Abstract Abstract
A collection of fungi was isolated from macroalgae of the genera Gracilaria, Enteromorpha and Ulva in the estuary Ria de Aveiro in Portugal. These isolates were characterized through a multilocus phylogeny based on ITS region of the ribosomal DNA, beta-tubulin (tub2) and translation elongation factor 1 alpha (tef1-α) sequences, in conjunction with morphological and physiological data. These analyses showed that the isolates represented an unknown fungus for which a new genus, Neptunomycesgen. nov. and a new species, Neptunomyces aureussp. nov. are proposed. Phylogenetic analyses supported the affiliation of this new taxon to the family Didymosphaeriaceae.
Keywords: Didymosphaeriaceae , marine fungi, phylogeny, taxonomy
Introduction
The family Didymosphaeriaceae is an important family in the order Pleosporales introduced by Munk (1953) and typified by the genus Didymosphaeria Fuckel with D. epidermidis as the type species. Members of this family are characterized by having brown 1-septate ascospores and trabeculate pseudoparaphyses that anastomose mostly above the asci (Aptroot 1995, Hyde et al. 2013, Ariyawansa et al. 2014a, b). Species of Didymosphaeriaceae are saprobes, endophytes or pathogens of a wide variety of plant species worldwide (Ariyawansa et al. 2014a, Liu et al. 2015, Wanasinghe et al. 2016).
Accurate species’ identification in genera of the family Didymosphaeriaceae was discussed in detail by Ariyawansa et al. (2014a). Phylogenetic analyses based on regions such as the internal transcribed spacer (ITS) region of the ribosomal DNA, beta-tubulin (tub2) and translation elongation factor 1 alpha (tef1-α) proved to be useful in delimiting taxa (Tennakoon et al. 2016, Ariyawansa et al. 2014b). Several studies have been conducted to resolve the boundaries of this family. First, Ariyawansa et al. (2014a) showed that Montagnulaceae and Didymosphaeriaceae were synonyms and thus, Ariyawansa et al. (2014b) synonymized Montagnulaceae under Didymosphaeriaceae and rearranged the family into 16 genera: Alloconiothyrium, Barria, Bimuria, Deniquelata, Didymocrea, Didymosphaeria, Julella, Kalmusia, Karstenula, Letendraea, Montagnula, Neokalmusia, Paraconiothyrium, Paraphaeosphaeria, Phaeodothis and Tremateia. Subsequently, in the last years, additional genera were added, namely Paracamarosporium and Pseudocamarosporium (Wijayawardene et al. 2014), Spegazzinia (Tanaka et al. 2015), Xenocamarosporium (Crous et al. 2015), Austropleospora and Pseudopithomyces (Ariyawansa et al. 2015) and Laburnicola and Paramassariosphaeria (Wanasinghe et al. 2016). More recently, Jayasiri et al. (2019) introduced Cylindroaseptospora and Gonçalves et al. (2019) reassigned the genus Verrucoconiothyrium previously included in the family Didymosphaeriaceae to the family Didymelaceae. Thus, the family Didymosphaeriaceae currently comprises 25 genera.
During an extensive survey of the fungal diversity from macroalgae species in the salt marsh of Ria de Aveiro in Portugal, we gathered a collection of fungal isolates. Here we report the morphological, cultural and phylogenetic characterization of these fungal isolates and introduce a novel genus and species to accommodate them.
Material and methods
Collection and isolation
Macroalgae (Gracilaria gracilis, Enteromorpha intestinalis, and other macroalgae species identified at genus-level only) were collected from various sites in the estuary Ria de Aveiro in Portugal (Table 1). Samples were placed in sterile plastic containers and maintained at 4 °C until fungal isolation. Algae samples were washed with autoclaved filtered saline water, cut into small pieces and placed on Potato Dextrose Agar (PDA) enriched with 3 % (w/v) sea salts (Sigma-Aldrich). Streptomycin and tetracycline, at final concentrations of 100 mg/L, were added to PDA to inhibit the growth of bacteria. From each sample (algae) 20 pieces of tissue were plated on PDA medium. The plates were incubated at 25 °C for 5 days and examined daily to observe the growth of fungal hyphae. Distinct fungal colonies were then transferred to new PDA plates for further isolation and purification.
Table 1.
Sampling sites.
| Locality name | GPS coordinates | Sampling date | Algae species collected |
|---|---|---|---|
| Ria de Aveiro | 40°37'45"N, 8°43'27"W | 26/09/18 | Ulva sp. |
| 40°39'33"N, 8°43'27"W | Enteromorpha sp. | ||
| 40°40'38"N, 8°42'20"W | Gracilaria gracilis, Ulva sp. | ||
| 40°43'00"N, 8°42'04"W | Enteromorpha intestinalis, Ulva sp. |
DNA isolation, amplification and analyses
Genomic DNA was extracted from fresh mycelium of cultures growing on PDA according to Möller et al. (1992). The primers ITS1 and ITS4 (White et al. 1990) were used for amplification and sequencing of the ITS region of the ribosomal DNA was as described by Alves et al. (2004). Beta-tubulin (tub2) gene was amplified and sequenced using T1 and Bt2b primers (Glass and Donaldson 1995, O’Donnell and Cigelnik 1997) with the cycling conditions previously described by Lopes et al. (2017). Translation elongation factor 1 alpha (tef1-α) gene was amplified and sequenced using EF1-688F and EF1-2218R primers (Rehner 2001, Alves et al. 2008). The amplified PCR fragments were purified with the NZYGelpure kit (NZYTech, Portugal) before sequencing at GATC Biotech (Cologne, Germany). The nucleotide sequences were analyzed with FinchTV v.1.4.0 (Geospiza Inc. www.geospiza.com/finchtv). A BLASTn search against the nucleotide collection (nr/nt) database using the ITS, tub2 and tef1-α sequences was carried out to determine the closest matching sequences, which were added to the sequence alignment. Sequences were aligned with ClustalX v. 2.1 (Thompson et al. 1997), using the following parameters: pairwise alignment parameters (gap opening = 10, gap extension = 0.1) and multiple alignment parameters (gap opening = 10, gap extension = 0.2, transition weight = 0.5, delay divergent sequences = 25 %). Alignments were checked and edited with BioEdit Alignment Editor v.7.2.5 (Hall 1999). Phylogenetic analyses were done with MEGA7 v.7.0 (Kumar et al. 2016). All gaps were included in the analyses. MEGA7 v.7.0 was also used to determine the best substitution model to be used to build the Maximum Likelihood (ML) tree. ML analysis was performed on a Neighbour-Joining (NJ) starting tree automatically generated by the software. Nearest-Neighbour-Interchange (NNI) was used as the heuristic method for tree inference with 1,000 bootstrap replicates. The sequences generated in this study were deposited in GenBank and taxonomic novelties in MycoBank. Alignment and tree were deposited in TreeBase (TB2:S24556).
Morphology and growth studies
Observations of morphological characters were made with a SMZ1500 stereoscopic microscope (Nikon, Japan) and a Nikon Eclipse 80i microscope (Nikon, Japan) equipped with differential interference contrast. Fungal structures were mounted in 100% lactic acid. Photographs and measurements were taken with a Nikon DSRi1 camera (Nikon, Japan) and the NIS-Elements D program (Nikon, Japan). Colony characters and pigment production were registered after 2 weeks of growth on PDA, Malt Extract Agar (MEA) and Oatmeal Agar (OA) incubated at 25 °C. Colony colors (obverse and reverse) were assessed according to the color charts of Rayner (1970). Morphological descriptions were based on cultures sporulating on PDA and pine needles, after 1-month incubation at 25 °C.
Temperature growth studies were performed for the new species described. A 5-mm diameter plug was taken from the margin of an actively growing colony (14-day-old) and placed in the center of PDA, MEA and OA plates. Three replicate plates per isolate were incubated at 10, 15, 20, 25, 30 and 35 °C in the dark. Colony diameter was measured after 1 and 2 weeks.
To evaluate the growth requirements for sea salts, the new species was cultured in PDA with 3% (w/m) sea salts. Three replicate plates per isolate were incubated at 25 °C for 2 weeks in the dark. After incubation the diameter of the colonies was measured and compared.
Results
Phenotype
Regarding conidial morphology, the fungal isolates studied were characterized by being aseptate and subcylindrical with rounded apices golden yellow conidia. For all media tested, the minimum, maximum and optimal growth temperatures were 10, 30 and 25 °C, respectively. No differences were observed in terms of colony diameter when grown in PDA with and without the addition of 3% sea salts, indicating that this fungus does not require salt for growth.
Phylogenetic analysis
BLASTn searches against the NCBI nucleotide database using the ITS, tub2 and tef1-α sequences of the isolates retrieved various hits, of which those with the highest sequence similarity belonged to members of the family Didymosphaeriaceae. Based on a megablast search using the ITS sequence, the closest matches for MUM 19.38 = CMG 10A in GenBank were Dothideomycetes sp. (GenBank accession: HQ631008; Identities 549/564 (97%), no gaps) and Letendraea sp. (GenBank accession: LT796897; Identities 548/564 (97%), no gaps). The closest hits using the tub2 sequence were Letendraea sp. (GenBank accession: LT796988; Identities 457/516 (89%), 5 gaps). Closest hits using tef1-α sequence also had highest similarity to Letendraea sp. (GenBank accession: LT797101; Identities 935/957 (98%), no gaps).
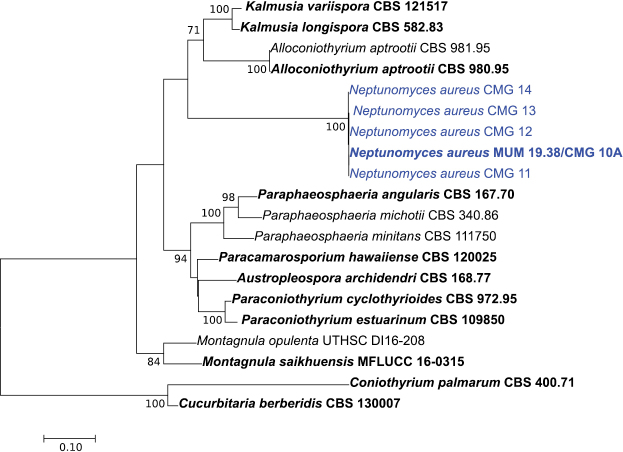
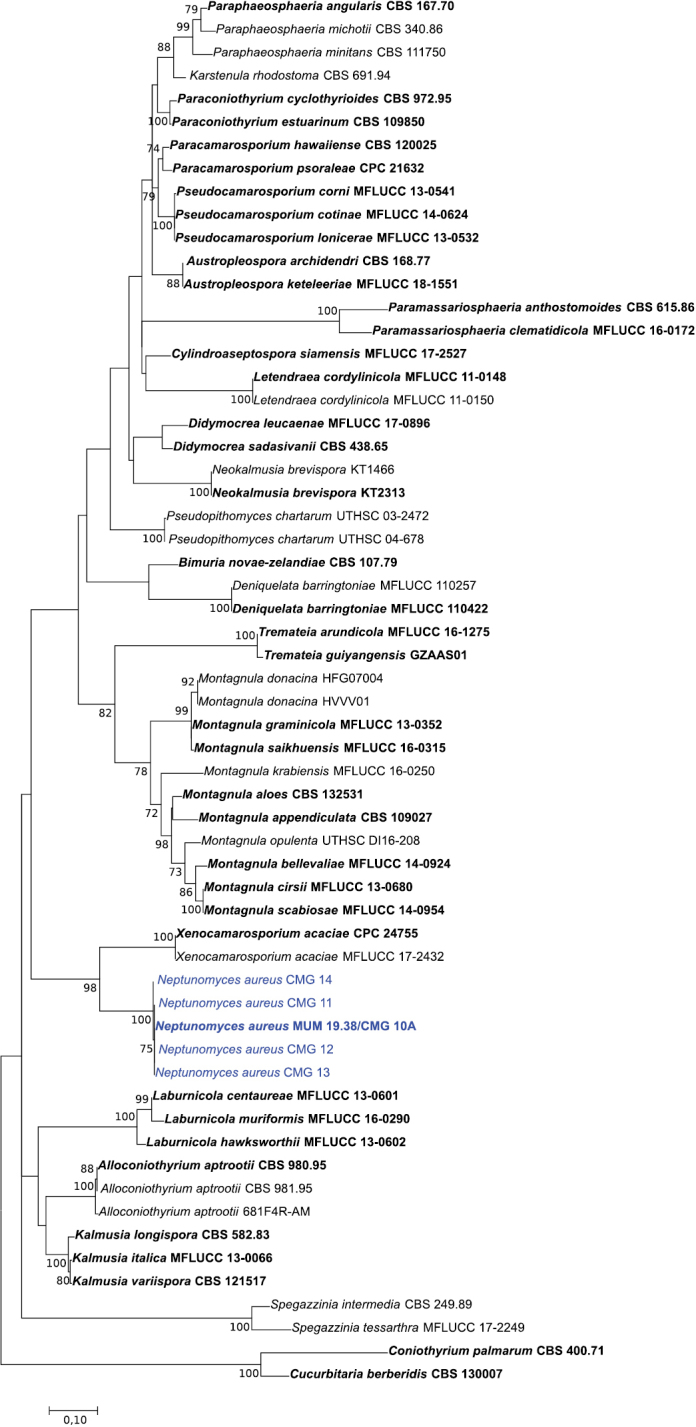
To confirm the phylogenetic placement of the fungal isolates within the family Didymosphaeriaceae, sequences of ITS, ITS + tub2 and ITS + tef1-α were aligned against those of several genera/species belonging to Didymosphaeriaceae (Suppl. material 1: Table S1). The alignment of the ITS, ITS + tub2 and ITS + tef1-α contained 60, 20 and 20 sequences (including the outgroup), and there was a total of 1010, 1352 and 1836 positions in the final dataset, respectively. In all ML phylogenetic trees (Figs 1–3), all novel isolates clustered in a monophyletic clade that received high (100 %) bootstrap support within the family Didymosphaeriaceae with a close relationship with the genera Alloconiothyrium and Kalmusia (ITS + tub2, Fig. 2) and Xenocamarosporium (ITS + tef1-α, Fig. 3). Thus, this novel lineage is phylogenetically well delimited, and it is clearly distinct from the other genera of Didymosphaeriaceae described so far and therefore it is proposed here as a new genus and a new species.
Figure 1.
Phylogenetic relationships of Didymosphaeriaceae species based on ITS sequence data and inferred using the Maximum Likelihood method under the Kimura 2-parameter model. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site and rooted to Cucurbitaria berberidis (CBS 130007) and Coniothyrium palmarum (CBS 400.71). Bootstrap values (> 70%) are shown at the nodes. Ex-type strains are in bold and the isolates from the current study are in blue.
Figure 3.
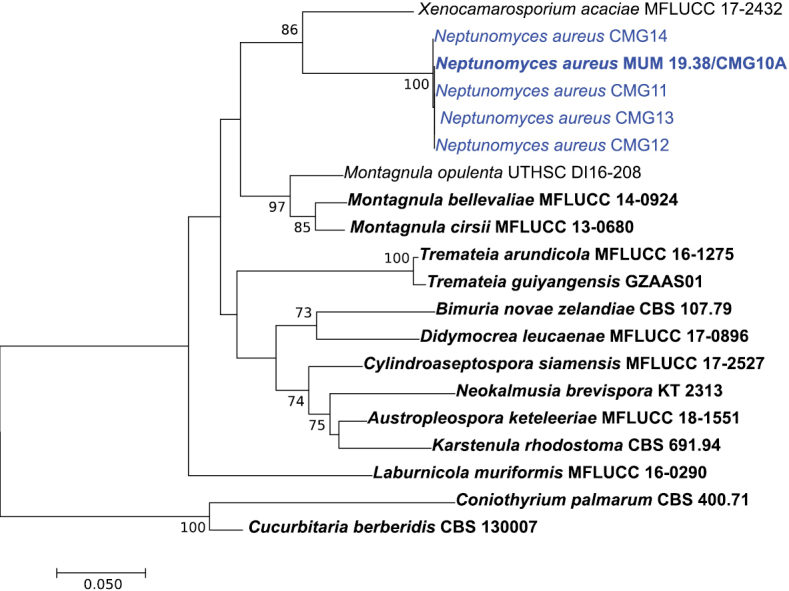
Phylogenetic relationships of Didymosphaeriaceae species based on ITS and tef1-α sequence data and inferred using the Maximum Likelihood method under the Kimura 2-parameter model. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site and rooted to Cucurbitaria berberidis (CBS 130007) and Coniothyrium palmarum (CBS 400.71). Bootstrap values (> 70%) are shown at the nodes. Ex-type strains are in bold and the isolates from the current study are in blue.
Figure 2.
Phylogenetic relationships of Didymosphaeriaceae species based on ITS and tub2 sequence data and inferred using the Maximum Likelihood method under the Kimura 2-parameter model. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site and rooted to Cucurbitaria berberidis (CBS 130007) and Coniothyrium palmarum (CBS 400.71). Bootstrap values (> 70%) are shown at the nodes. Ex-type strains are in bold and the isolates from the current study are in blue.
Taxonomy
Neptunomyces
M. Gonçalves, T. Vicente & A. Alves. Portugal gen. nov.
90966B60-2CC7-53F6-AA0B-D9C495E39648
831436
Description.
Asexual morph: mycelium consisting of septate, smooth hyphae, thick-walled, hyaline and rarely with nucleus. Conidia aseptate, golden yellow, smooth, subcylindrical with rounded apices. Chlamydospores not observed. Sexual morph unknown.
Etymology.
Referring to Neptune (Latin: Neptūnus) the god of the seas in Roman mythology.
Type species.
Neptunomyces aureus M. Gonçalves, T. Vicente & A. Alves. Portugal
Neptunomyces aureus
M. Gonçalves, T. Vicente & A. Alves. Portugal sp. nov.
68ED7DCA-10D4-5A69-AEC1-6A277A9900DA
831437
Figure 4.
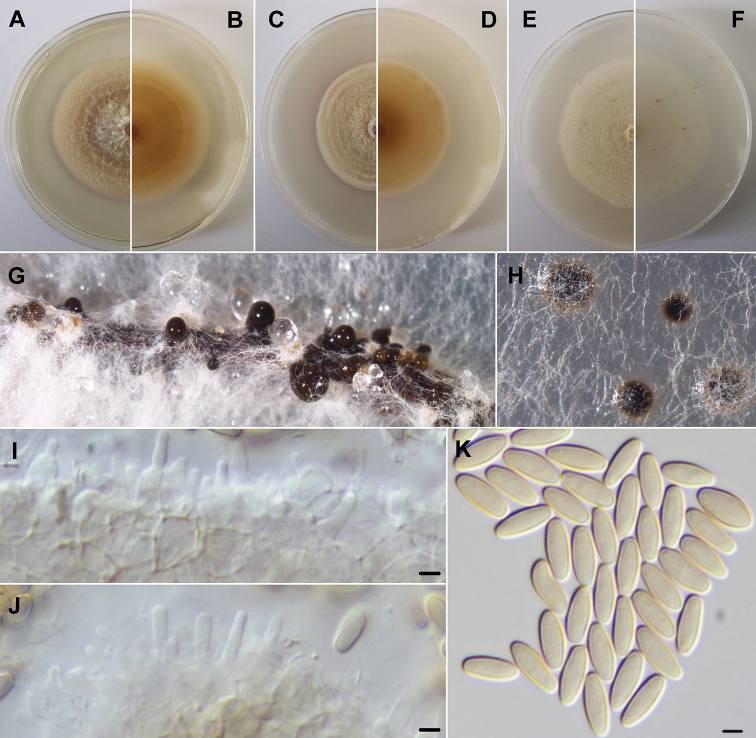
Neptunomyces aureus (MUM 19.38). A, B Colony after 2 weeks at 25 °C on PDA (obverse and reverse) C, D colony after 2 weeks at 25 °C on MEA (obverse and reverse) E, F colony after 2 weeks at 25 °C on OA (obverse and reverse) G, H conidiomata after 1 month at 25 °C on pine needles and PDA. I, J conidiogenous cells K conidia. Scale bars: 2.5 μm.
Type.
Portugal, Ria de Aveiro (40°40'38"N, 8°42'21"W), isolated from Gracilaria gracilis, 26th September 2018, M. Gonçalves, (holotype: a dried culture sporulating on pine needles AVE-F-1; ex-type living culture, MUM 19.38 = CMG 10A).
Etymology.
Referring to the golden yellow conidia.
Diagnosis.
Phylogenetic analysis based on the ITS, ITS and tub2 and ITS and tef1-α dataset considered in the present study clustered the retrieved strains in a monophyletic lineage in the family Didymosphaeriaceae. Therefore, a new genus Neptunomyces gen. nov., and a new species Neptunomyces aureus sp. nov. are here proposed.
Description.
Mycelium smooth, white, 2–3 μm wide hyphae. Hyphae thick-walled, smooth, hyaline and rarely with nucleus. Conidiomata aggregated or solitary, globose to subglobose, dark brown, immersed or rarely superficial. Conidiomata wall pseudoparenchymatous. Conidiophores reduced to ampulliform to subcylindrical, hyaline, smooth conidiogenous cells (mean ± S.D. = 5.2 ± 0.3 × 2.0 ± 0.6 μm, n = 20). Conidia solitary, subcylindrical with rounded apices, aseptate, initially hyaline, smooth, becoming golden yellow (mean ± S.D. = 7.0 ± 0.6 × 2.7 ± 0.2 μm, n = 100). Sexual morph unknown.
Culture characteristics.
On 2 weeks old PDA and OA plates, at 25 °C, colonies growing to 50 mm in diameter, regular and above and a little immersed into agar. PDA obverse white near the center getting flesh orange towards the borders; reverse buff orange in the center and lighter in periphery. OA obverse skimmed milk white; reverse snow white. On 2 weeks old MEA plates, at 25 °C, colonies growing to 44 mm in diameter, regular and above and a little immersed into agar. Obverse orange-colored white; reverse reddish orange in the center and ochre yellow in periphery. At 35 °C, there was no growth in any media tested.
Distribution.
Estuary Ria de Aveiro, Portugal
Additional specimens examined.
Portugal, Ria de Aveiro (Table 1), isolated from Ulva sp., Enteromorpha intestinalis and Enteromorpha sp. (Supp. material 1: Table S1). M. Gonçalves, living cultures CMG 11, CMG 12, CMG 13 and CMG 14.
Notes.
Neptunomyces aureus clustered in a distinct lineage in the family Didymosphaeriaceae with high p-distances (= 0.07) of nucleotide sites among the two-loci sequences (ITS and tef1-α) with closest genus Xenocamarosporium. Although the morphology of conidiomata, conidiomata wall and conidiogenous cells can be very similar in the genera of this family, conidial morphology distinguishes Neptunomyces from Xenocamarosporium (Table 2).
Table 2.
Comparison of Neptunomyces aureus and Xenocamarosporium acaciae.
| Species | Neptunomyces aureus | Xenocamarosporium acaciae | |
|---|---|---|---|
| Strain | MUM 19.38 | CBS 139895 | |
| Nucleotide differences | ITS | 65 | |
| tef1-α | 58 | ||
| (p-distance) | ITS + tef1-α | 0.07 | |
| Conidia | Size (μm) | 7.0 ± 0.6 × 2.7 ± 0.2 | (11–)12–14(–15) × (3.5–)4(–5) |
| Morphology | Subcylindrical | Ellipsoidal to subcylindrical | |
| Apex and base | Rounded | Obtuse and rounded to truncate base | |
| Color | Hyaline becoming golden yellow | Hyaline becoming golden brown | |
| Septation | Aseptate | (1–)3-septate | |
| Conidiogenous cells | Size (μm) | 5.2 ± 0.3 × 2.0 ± 0.6 | 7–12 × 5–7 |
| Morphology | Ampulliform | Ampulliform | |
| Color | Hyaline | Hyaline | |
| References | Present study | Crous et al. 2015 | |
Discussion
This study adds to the family Didymosphaeriaceae a new genus/species, namely Neptunomyces aureus isolated from macroalgae in the estuary of Ria de Aveiro in Portugal. The family Didymosphaeriaceae contains now 26 genera described.
The majority of the genera in the Didymosphaeriaceae remain under studied, which makes the family still poorly understood and not well resolved (Wanasinghe et al. 2016). In fact, there was no β-tubulin and tef1-α sequence data available for many species and therefore the phylogenetic analyses presented did not encompass all known species of the family. For example, phylogenetic analyses based on ITS + tub2 revealed that N. aureus is closely related to the genera Alloconiothyrium and Kalmusia, while on ITS + tef1-α it is related to the genus Xenocamarosporium, since there is no tef1-α/tub2 for Alloconiothyrium, Kalmusia and Xenocamarosporium, respectively. However, this family contains several well supported clades, most of which correspond to monotypic genera (e.g. Alloconiothyrium, Bimuria, Karstenula, Xenocamarosporium), or genera with only two species (e.g. Cylindroaseptospora, Deniquelata, Didymocrea).
Comparison of the ITS and tef1-α sequences from N. aureus and the closest genus/species X. acaciae revealed 65 and 58 base pair differences, respectively, with high p-distances (= 0.07) supporting the establishment of Neptunomyces as a distinct genus. Although the morphology of conidiomata, conidiomata wall and conidiogenous cells are similar, the conidiogenous cells of N. aureus are smaller than those of X. acaciae. Also, both can be easily discriminated by their conidia morphology, color and size. The conidia of N. aureus are aseptate, subcylindrical with rounded apices and initially hyaline and soon become golden yellow, while conidia of X. acaciae are mostly tri-septate, ellipsoidal to subcylindrical, sometimes with truncate base and golden brown. Moreover, conidia of N. aureus are considerably smaller than those of X. acaciae.
Neptunomyces aureus was isolated from healthy tissues of the macroalgae analyzed, where it may occur as endophyte or epiphyte. Further investigations are essential for clarifying its biology, ecology, physiological characteristics and host-specificity. Moreover, we did not obtain any sexual morph for this new species and there is no molecular support to link possible sexual taxa.
So far, species of Didymosphaeriaceae seem to be cosmopolitan in distribution: they have been recorded from both temperate and tropical regions. Also, Didymosphaeriaceae have been found on various hosts and substrates, including plants, humans and soil, being regarded as saprobes, endophytes or pathogens of a wide variety of plant substrates worldwide (Ariyawansa et al. 2014a, Liu et al. 2015, Wanasinghe et al. 2016). However, most Didymosphaeriaceous genera occur on plants of more than 20 host families, the majority of them being monocotyledons and herbaceous plants, such as Anacardiaceae, Asparagaceae, Asteraceae, Caprifoliaceae, Euphorbiaceae, Fagaceae, Lecythidaceae and Poaceae. Reports of Didymosphaeriaceous species in marine/estuarine environments are almost non-existent. So far, this new genus/species has been found only in association with macroalgae species. Garzoli et al. (2018) reported, for the first time, some species within this family in Padina pavonica, a brown alga collected in the Mediterranean Sea: Paraconiothyrium variabile, Paraphaeosphaeria neglecta and another eight unidentified Didymosphaeriaceous species. Also, Paraconiothyrium estuarinum was isolated from sediments of an estuarine environment (Verkley et al. 2004) and Paraphaeosphaeria michotii from Phragmites australis, also typically found in estuaries (Eriksson 1967).
Physiological tests allowed us to characterize the retrieved isolates as a slight halophile as they grow equally well in the presence and absence of 3% sea salts. Information regarding NaCl tolerance is still poorly described in Didymosphaeriaceous species, but future studies related to tolerance to salinity in these organisms (especially in this new species) may provide physiological unique characteristics which may have some biotechnological potential.
Supplementary Material
Acknowledgements
The authors acknowledge financial support from the Portuguese Foundation for Science and Technology (FCT) to CESAM (UID/AMB/50017/2019) and the PhD grant of M. Gonçalves (SFRH/BD/129020/2017).
Citation
Gonçalves MFM, Vicente TFL, Esteves AC, Alves A (2019) Neptunomyces aureus gen. et sp. nov. (Didymosphaeriaceae, Pleosporales) isolated from algae in Ria de Aveiro, Portugal. MycoKeys 60: 31–44. https://doi.org/10.3897/mycokeys.60.37931
Supplementary materials
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Micael F.M. Gonçalves, Tânia F.L. Vicente, Ana C. Esteves, Artur Alves
Table S1. List of isolates used in this study
Data type: species data
References
- Alves A, Crous PW, Correia A, Phillips AJL. (2008) Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Diversity 28: 1–13. [Google Scholar]
- Alves A, Correia A, Luque J, Phillips AJL. (2004) Botryosphaeria corticola sp. nov. on Quercus species, with notes and description of Botryosphaeria stevensii and its anamorph Diplodia mutila. Mycologia 96: 598–613. 10.1080/15572536.2005.11832956 [DOI] [PubMed] [Google Scholar]
- Aptroot A. (1995) Redisposition of some species excluded from Didymosphaeria (Ascomycotina). Nova Hedwigia 60: 325–379. [Google Scholar]
- Ariyawansa HA, Camporesi E, Thambugala KM, Mapook A, Kang J, Alias S, Chukeatirote E, Thines M, Mckenzie E, Hyde KD. (2014a) Confusion surrounding Didymosphaeria phylogenetic and morphological evidence suggest Didymosphaeriaceae is not a distinct family. Phytotaxa 176: 102–119. 10.11646/phytotaxa.176.1.12 [DOI] [Google Scholar]
- Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe D, Mapook A, Chukeatirote E, Kang J, Xu J, McKenzie E, Jones E, Hyde KD. (2014b) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae). Fungal Diversity 68: 69–104. 10.1007/s13225-014-0305-6 [DOI] [Google Scholar]
- Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lücking R, Ghobad-Nejhad M, Niskanen T, Thambugala KM, Voigt K, Zhao RL, Li GJ, Doilom M, Boonmee S, Yang ZL, Cai Q, Cui YY, Bahkali AH, Chen J, Cui BK, Chen JJ, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, Hashimoto A, Hongsanan S, Jones EBG, Larsson E, Li WJ, Li QR, Liu JK, Luo ZL, Maharachchikumbura SSN, Mapook A, McKenzie EHC, Norphanphoun C, Konta S, Pang KL, Perera RH, Phookamsak R, Phukhamsakda C, Pinruan U, Randrianjohany E, Singtripop C, Tanaka K, Tian CM, Tibpromma S, Abdel-Wahab MA, Wanasinghe DN, Wijayawardene NN, Zhang JF, Zhang H, Abdel-Aziz FA, Wedin M, Westberg M, Ammirati JF, Bulgakov TS, Lima DX, Callaghan TM, Callac P, Chang CH, Coca LF, Dal-Forno M, Dollhofer V, Fliegerová K, Greiner K, Griffith GW, Ho HM, Hofstetter V, Jeewon R, Kang JC, Wen TC, Kirk PM, Kytövuori I, Lawrey JD, Xing J, Li H, Liu ZY, Liu XZ, Liimatainen K, Thorsten Lumbsch H, Matsumura M, Moncada B, Nuankaew S, Parnmen S, Santiago ALCMDA, Sommai S, Song Y, de Souza CAF, de Souza-Motta CM, Su HY, Suetrong S, Wang Y, Fong WS, Yuan HS, Zhou LW, Réblová M, Fournier J, Camporesi E, Luangsa-ard JJ, Tasanathai K, Khonsanit A, Thanakitpipattana D, Somrithipol S, Diederich P, Millanes AM, Common RS, Stadler M, Yan JY, Li XH, Lee HW, Nguyen TTT, Lee HB, Battistin E, Marsico O, Vizzini A, Vila J, Ercole E, Eberhardt U, Simonini G, Wen HA, Chen XH, Miettinen O, Spirin V. (2015) Fungal Diversity Notes 111–252 – taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 75: 27–274. 10.1007/s13225-015-0346-5 [DOI] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, Hernandez-Restrepo M, Sutton DA, Acharya K, Barber PA, Boekhout T, Dimitrov RA, Dueñas M, Dutta AK, Gené J, Gouliamova DE, Groenewald M, Lombard L, Morozova OV, Sarkar J, Smith MT, Stchigel AM, Wiederhold NP, Alexandrova AV, Antelmi I, Armengol J, Barnes I, Cano-Lira JF, Castañeda Ruiz RF, Contu M, Courtecuisse PR, da Silveira AL, Decock CA, de Goes A, Edathodu J, Ercole E, Firmino AC, Fourie A, Fournier J, Furtado EL, Geering AD, Gershenzon J, Giraldo A, Gramaje D, Hammerbacher A, He XL, Haryadi D, Khemmuk W, Kovalenko AE, Krawczynski R, Laich F, Lechat C, Lopes UP, Madrid H, Malysheva EF, Marín-Felix Y, Martín MP, Mostert L, Nigro F, Pereira OL, Picillo B, Pinho DB, Popov ES, Rodas Peláez CA, Rooney-Latham S, Sandoval-Denis M, Shivas RG, Silva V, Stoilova-Disheva MM, Telleria MT, Ullah C, Unsicker SB, van der Merwe NA, Vizzini A, Wagner HG, Wong PT, Wood AR, Groenewald JZ. (2015) Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. 10.3767/003158515X688433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson O. (1967) On graminicolous pyrenomycetes from Fennoscandia I. Dictyosporous species (339–380). II. Phragmosporous and scolecosporous species (381–440). III. Amerosporous and didymosporous species (441–466). Arkiv før Botanik 6: 339–466. [Google Scholar]
- Garzoli L, Poli A, Prigione V, Gnavi G, Varese GC. (2018) Peacock’s tail with a fungal cocktail: first assessment of the mycobiota associated with the brown alga Padina pavonica. Fungal Ecology 35: 87–97. 10.1016/j.funeco.2018.05.005 [DOI] [Google Scholar]
- Glass NL, Donaldson G. (1995) Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves MFM, Silva BM, Esteves AC, Alves A. (2019) Verrucoconiothyrium ambiguum sp. nov., a novel species isolated from sea water, and affiliation of the genus Verrucoconiothyrium to the family Didymellaceae International Journal of Systematic and Evolutionary Microbiology. 10.1099/ijsem.0.003680 [DOI] [PubMed]
- Hall TA. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu Y-X, Lucking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, De Hoog S, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zhang M. (2013) Families of Dothideomycetes. Fungal Diversity 63: 1–313. 10.1007/s13225-013-0263-4 [DOI] [Google Scholar]
- Jayasiri SC, Hyde KD, Jones EBG, McKenzie EHC, Jeewon R, Phillips AJL, Bhat DJ, Wanasinghe DN, Liu JK, Lu YZ, Kang JC, Xu J, Karunarathna SC. (2019) Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 10: 1–186. 10.5943/mycosphere/10/1/1 [DOI] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, Hyde KD, Jones EBG, Ariyawansa HA, Bhat JD, Boonmee S, Maharachchikumbura SSN, Mckenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, D’souza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathana SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipol S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wuayawardene NN, Wanasinghe D, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Li XL, Liu XX, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sriindrasutdhi V, Tian CM, Verbeken A, Von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E. (2015) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Diversity 72: 1–197. 10.1007/s13225-015-0324-y [DOI] [Google Scholar]
- Lopes A, Phillips AJL, Alves A. (2017) Mating type genes in the genus Neofusicoccum: Mating strategies and usefulness in species delimitation. Fungal Biology 121: 394–404. 10.1016/j.funbio.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Möller EM, Bahnweg G, Sandermann H, Geiger HH. (1992) A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Research 20: 6115–6116. 10.1093/nar/20.22.6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk A. (1953) The system of the pyrenomycetes. A contribution to a natural classification of the group Sphaeriales sensu Lindau. Dansk Bot Ark 15: 1–163. [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute.
- Rehner SA. (2001) Primers for elongation factor 1-α (EF1-α). http://www.nacse.org/yfaaberg/aftol/EF1primer.pdf
- O’Donnell K, Cigelnik E. (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. 10.1006/mpev.1996.0376 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y, Shirouzu T, Hosoya T. (2015) Revision of the Massarineae (Pleosporales, Dothideomycetes). Studies in Mycology 82: 75–136. 10.1016/j.simyco.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennakoon DS, Hyde KD, Wanasinghe DN, Bahkali AH, Camporesi E, Khan S, Phookamsak R. (2016) Taxonomy and phylogenetic appraisal of Montagnula jonesii sp. nov. (Didymosphaeriaceae, Pleosporales) from Italy. Mycosphere 7: 1346–1356. 10.5943/mycosphere/7/9/8 [DOI] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkley GJ, da Silva M, Wicklow DT, Crous PW. (2004) Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Studies in Mycology 50: 323–335. [Google Scholar]
- Wanasinghe DN, Jones EBG, Camporesi E, Dissanayake AJ, Kamolhan S, Mortimer PE, Xu J, Abd-Elsalam KA, Hyde KD. (2016) Taxonomy and phylogeny of Laburnicola gen. nov. and Paramassariosphaeria gen. nov. (Didymosphaeriaceae, Massarineae, Pleosporales). Fungal Biology 120: 1354–1373. 10.1016/j.funbio.2016.06.006 [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal genes for phylogenies. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR Protocols: A Guide to Methods and Applications.Academic Press, California, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Wijayawardene NN, Hyde KD, Bhat DJ, Camporesi E, Schumacher RK, Chethana KWT, Wikee S, Bahkali AH, Wang Y. (2014) Camarosporium-like species are polyphyletic in Pleosporales; introducing Paracamarosporium and Pseudocamarosporium gen. nov. in Montagnulaceae. Cryptogamie Mycologie 35: 177–198. 10.7872/crym.v35.iss2.2014.177 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Micael F.M. Gonçalves, Tânia F.L. Vicente, Ana C. Esteves, Artur Alves
Table S1. List of isolates used in this study
Data type: species data