Abstract
Light is an important factor that can induce the growth of varieties of organisms including fungi and their secondary metabolites. The evolutions of biomass, carotenoids, lipid production, compositions and contents of fatty acid and amino acid in Rhodotorula mucilaginosa were investigated under different light irradiation conditions. The results indicated that irradiation with 1700 lx could promote the growth and glucose assimilation of R. mucilaginosa, compared to the dark control, while the trial with 3500 lx had certain inhibiting effects. The carotenoids concentrations and percentages of unsaturated fatty acid (USFA, C16:1 and C18:1) increased with the improvement of irradiation intensity. Conversely, the proportions of saturated fatty acids (C16:0, C18:0 and C20:0) were decreased. The relative contents of amino acid and total protein were reduced under illumination compared to dark control. Conclusively, irradiation could change the cell growth and metabolites of the pigmented fungus, which implied that there may be a photoinduced mode exists in R. mucilaginosa similar to that of Neurospora crassa, and it also could be applied to regulate the biosynthesis and production of valuable components such as carotenoids and USFA.
Keywords: Rhodotorula mucilaginosa, Light irradiation, Growth characteristics, Metabolites compositions, Regulation
Introduction
Experimentally, it has been found that light irradiation can induce the growth and development of varieties of organisms including fungi and their secondary metabolites such as carotenoids (Rodriguez-Romero et al. 2010; Llorente et al. 2017). For instance, light irradiation can induce the production of carotenoids biosynthesis in Phycomyces blakesleeanus (Rua et al. 1987). The effects of light irradiation on fungi are mainly influenced by light intensity, quality and color. It is reported that the synthesis of carotenoids and the formation of spores in Neurospora crasa (Morelli et al. 1993) were affected by light. Wang et al. (2012) reported that the influence of blue light on citrinin biosynthetic gene expression in Monascus, and the citrinin production increased from 478 to 698 mg/L when Monascus MX was grown under blue light instead of in the dark. Babitha et al. (2008) investigated the effect of light quality on growth and pigment yield of Monascus purpureus and found that incubation in total darkness increased red pigment production from 14.5 to 22 OD/g, in contrast, the growth of the fungus under a direct illumination resulted in total suppression of pigment production. The authors proposed the existence of a light-perception system in Monascus purpureus according to the above results. Therefore, light irradiation is an effective way to regulate the secondary metabolites of fungi.
Rhodotorula genus is a heterotrophic organism and pigmented yeast, part of the Basidiomycota phylum, particularly important for food, bioconversion, and bioenergy industries because of its biotechnological potential and safety implications (Hernández-Almanza et al. 2014; Saenge et al. 2011). Various strains of Rhodotorula present important features such as the production of large amounts of carotenoids, single-cell proteins and microbial lipids (Dworecka-Kaszak and Kizerwetter-Swida 2011). Some species of Rhodotorula are the main carotenoid producing microorganisms with a predominant synthesis ability of β-carotene, torulene, and torularhodin (Marova et al. 2012). In addition, the total oil content, lipid composition, and carotenoid components have also been analyzed, which led to a closer understanding of Rhodotorula (Perrier et al. 1995). As a potential biotechnological microorganism, Rhodotorula has been studied for many years. Physiological studies have also revealed interesting features as industrial yeast, such as the capacity to grow to high cell density and its ability to utilize a wide range of carbon and nitrogen sources (Park et al. 2018). The effects of light on the growth kinetics and metabolism of Rhodotorula were also found in works related to its physiological metabolism. The dry weight of β-carotene, torulene and rhodopsin of R. glutinis were reported to be slightly increased by weak light irradiation, more significantly, the yields of rhodopsin improved from 7.9 to 14.2 mg/100 g (Sakaki et al. 2001). The carotenoid accumulation in Pestalotiopsis sp. F was also induced by blue light and increased following with the extension of irradiation time (Fu and Zou 2009).
So far, there have been some reports as above focusing on the effects of light on growth, physiological and biochemical characteristics of Phycomyces, Neurospora and Monascus, however, there is little intensive work on the photobiology of Rhodotorula, which is an interesting question about the photobiology of non-photosynthetic fungi. In the present study, a carotenoid producing strain with orange red color was screened from soil and identified as Rhodotorula mucilaginosa. Furthermore, the effects of different light intensity treatments on the growth kinetics, glucose consumption, contents of carotenoids and lipids, composition of fatty acid and amino acid were investigated. The aims of this work were to find out whether light irradiation could play a role in the growth of heterotrophic yeast, R. mucilaginosa, and to find the effects of irradiation on the growth characteristics and metabolites composition for biosynthesis regulation of functional components in the fungus.
Materials and methods
Strain and growth conditions
The strain of R. mucilaginosa (K-1) was isolated and screened from soil and identified using morphological, physiological, biochemical tests and rDNA-ITS sequence analysis. During cultivation, the colony color gradually changed from white to orange red, with bright surface, soft and sticky texture. There was no obvious change in medium color and no exudate. The strain is preserved in the author’s lab.
The fermentation medium was comprised of defined amounts (g/L) of glucose 40, yeast extract 1.5, (NH4)2SO4 2, KH2PO4 1, MgSO4·7H2O 0.5, CaCl2 0.1, and NaCl0.1, pH = 5.5. All media were sterilized 15 min at 121 °C and 0.1 MPa.
Ten milliliters of prepared seed suspension of R. mucilaginosa was inoculated in 90 mL of YEPD liquid medium (10 g/L of yeast extract, 20 g/L of peptone, 20 g/L of glucose). It was used as seed culture after shaking at 150 rpm and cultivated at 30 ± 1 °C for 24 h under aerobic conditions. Then, 5 mL of the seed (5%) was transferred into 250 mL flasks containing 95 mL medium and cultured at 150 rpm and 30 ± 1 °C for 168 h (7 days). At the end of culture, the flask broth of R. mucilaginosa was collected and centrifuged, freeze-dried cells were used as samples for lipid, carotenoids and amino acids extraction and analysis.
Light exposure conditions
Two lighting shakers (ZWYR-211D, Shanghai) were constructed to enable incubation of cultures under different intensities of white bio-lamp. According to the intensity level of the light source and our pretest results, each shaker was equipped with the following conditions: shaker 1, 1700 lx; shaker 2, 3500 lx. Another shaker with dark condition was set as a control. The light cycle of the two illumination groups was set as 12 h dark: 12 h light, the sampling and analysis were carried out every 24 h intervals.
Measurement of growth kinetic parameters and dry biomass
Five milliliters medium at different culture conditions and groups was sampled and centrifuged for 10 min at 5000 rpm. Then, the residual glucose content and pH in the supernatant was measured, while the precipitated cells were washed twice with distilled water and centrifuged again as above. The centrifuge tube containing wet cell was dried at 105 °C to constant and quantified gravimetrically after cooling in a desiccator to room temperature. The dry biomass concentration was measured as Eq. (1):
| 1 |
where C is the biomass concentration (g/L), Wt and Wc are the dry weight of tube containing dried cell (g) and blank tube dry weight (g), respectively. And V is the sampling broth volume (mL).
The specific growth rate and productivity of R. mucilaginosa were also tested as Eq. (2) and (3):
| 2 |
| 3 |
where μ is the specific growth velocity (day−1), W1 and W0 are the dry cell weight in the exponential phase and initial exponential phase on days t1 and t0, respectively. P is the productivity of R. mucilaginosa, (Andrade and Costa 2007; Malisorn and Suntornsuk 2009; Kong et al. 2013).
Detection of glucose concentration and pH in medium
The residual glucose concentration in the supernatant of culture medium after centrifugation was detected by 3, 5-dinitrosalicylic acid colorimetric method (Miller 1959). The pH in the culture medium was assayed using a pH detector (PHS-3C, Shanghai).
Extraction and determination of total carotenoids
The extraction and analysis of carotenoids was following the modified methods described in literature (Malisorn and Suntornsuk 2009; Buzzini and Martini 2000). In brief, the disrupted cell sample using a grinder (Scientz-50, Beijing) was extracted with acetone three times, and all the extracted supernatant was mixed, metered volume and assayed at 486 nm with a UV–VIS spectrophotometer (UV-1800, Shimadzu). The content of total carotenoids was calculated as Eq. (4).
| 4 |
where A is the absorbance at 486 nm, D is the dilution ratio, V is the total volume of sample, 0.16 is extinction coefficient of carotenoids, W is dry cell weight.
Extraction and analysis of total lipids and fatty acid compositions
The total lipids were extracted from frozen dried and disrupted biomass powder by the chloroform–methanol method and gravimetrically quantified using previously optimized method (Folch et al. 1957; Bligh and Dyer 1959). The fatty acid methyl-ester sample was prepared according to the standard method of GBT17376-2008. The fatty acid components were analyzed by Gas-Chromatograph and Mass Spectrometry (GC–MS, 7890A/5975C, Agilent). The operation parameters of GC–MS analysis were described as follows: RTX-5MS elastic quartz capillary: 30 m (length) × 0.25 mm (inner diameter) × 0.25 μm (thickness), transmission line temperature: 250 °C, inlet temperature: 250 °C, the ion source temperature: 250 °C, electron energy: 70 eV, scanning range: 50-650 amu.
Analysis of amino acid compositions
Amino acid compositions of the freeze-dried sample of R. mucilaginosa biomass were measured by the standard method of GB 5009.124-2016, except for cysteine and tryptophan. Tryptophan was determined according to GB/T 15400-1994. Cysteine content was tested using Fluorometric Cysteine Assay Kit.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) from triplicate for biomass cultivation, metabolites extraction and determination. The Analysis of Variance (ANOVA) was applied to the results, followed by the Tukey’s test at 95% confidence level by SPSS 13.0, and OriginPro 8.5 software was used to draw figures.
Results and discussion
Effects of different light irradiation on growth and biomass productivity
To observe the influence of different light intensity treatment on the growth of R. mucilaginosa, we inoculated the fungus in liquid medium at 30 °C under three different light irradiation conditions (dark, 1700 lx and 3500 lx). A significant difference (p < 0.05) was observed from the growth curve of the cultivation under 1700 lx illumination compared with dark and 3500 lx groups (Fig. 1). After seven days of cultivation in a liquid medium, the biomass of dark, 1700 and 3500 lx groups reached 6.64 ± 0.10 g/L, 7.88 ± 0.13 g/L, and 6.37 ± 0.22 g/L, respectively. The biomass concentration, specific growth rate (2.32 ± 0.17 day−1) and biomass productivity (1.28 ± 0.02 g/L day) of R. mucilaginosa in 1700 lx group were higher than dark and 3500 lx groups. It also could be seen from Fig. 1 that the growth trend in 1700 lx trial obviously higher than the experimental groups of dark and 3500 lx. The results indicated that appropriate light irradiation significantly enhanced the growth of R. mucilaginosa, however, strong light intensity could inhibit the fungus growth. According to the report from Sakaki et al. (2001), irradiation at 3500 lx was not sufficiently strong to affect the growth of Saccharomyces cerevisiae, but the growth of R. glutinis was slightly inhibited by light irradiation under the same conditions with a delay of the growth in the logarithmic growth phase. However, irradiation could improve the maximum biomass achieved and specific growth rate of R. glutinis in a batch culture with 2 or 3 LED lights, compared to a batch without irradiation (Yen and Zhang 2011). These results implied that the light-induced growth might be a common phenomenon in genes of Rhodotorula with the capacity of pigments biosynthesis (Schreiber et al. 1982). The molecular regulation mechanism of growth promotion induced by light in R. mucilaginosa need to be further investigated.
Fig. 1.
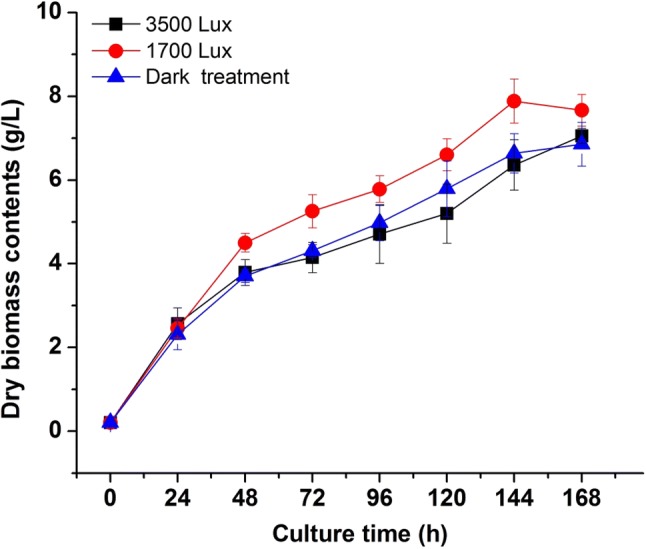
Effects of different light irradiation on the growth curve of R. mucilaginosa
Besides, glucose content and pH in culture medium were measured under different light intensities. As shown in Fig. 2a and b, the consumption of glucose and pH changes in liquid media were affected by different light intensity treatments. With the increase of biomass concentration of R. mucilaginosa, the glucose contents in culture media decreased dramatically (Fig. 2a). The glucose decrease rate in 1700 lx group was faster than groups of 3500 lx and dark. The content of residual glucose in 1700 lx group was the lowest (from 40 to 10.4 g/L), while the glucose in dark and 3500 lx groups were 14.3 g/L and 15.8 g/L, respectively. The results indicated that strong light intensity not only reduced the biomass production of R. mucilaginosa, but also decreased the glucose assimilation. Nevertheless, suitable light stimulation could promote the utilization of glucose and cell growth of R. mucilaginosa. The pH curves in different media also showed the relationships between biomass production, glucose consumption and pH changes under different light treatment (Fig. 2b). The change and difference of pH in medium might be due to the glucose assimilation and organic acids generation during fermentation of R. mucilaginosa. Above results suggested that different intensity of light irradiation could influence the growth dynamics and physiological metabolism.
Fig. 2.
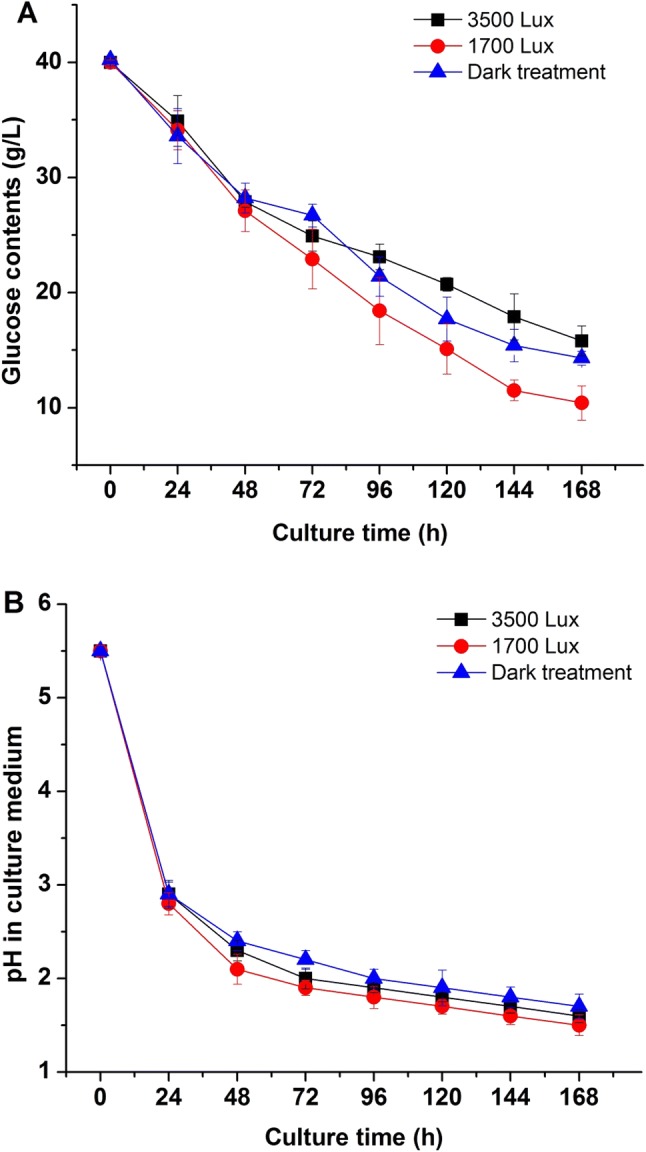
Effects of different light irradiation on the glucose consumption (a) and pH (b) in culture medium of R. mucilaginosa
Light regulates fungal development and behaviour and activates metabolic pathways. Moreover, light is one of the many signals that fungi use to perceive and interact with the environment (Corrochano 2007). The ascomycete Neurospora crassa has been widely used as a model to understand the molecular mechanisms of fungal responses to light (Dasgupta et al. 2015). The responses to light in Neurospora are mediated by the activity of a light-regulated transcription factor complex, the white collar complex, and result in light-regulated changes in gene transcription (Olmedo et al. 2013). Although there are many reports related to the light regulation of the growth and metabolite of Rhodotorula (Tada and Shiroishi1982; Schreiber et al. 1982; Yen and Zhang 2011), the work on the regulation mechanism is rare. Our results suggest that there might be photoregulatory factors exist in Rhodotorula similar to those of Neurospora (Olmedo et al. 2018), which is meaningful to study in the future.
Effects of different light irradiation on carotenoids production
As shown in Fig. 3, the different light intensities affected the production of carotenoids by R. mucilaginosa significantly. In the initial 48 h, the groups (1700 and 3500 lx) with illumination had higher contents than the dark group, which proved that light treatment could stimulate the pigments biosynthesis. After 24 h of culture, the cell concentration in the 1700 lx group was higher than that in the dark and 3500 lx groups (Fig. 1). However, the relatively higher carotenoid concentration was achieved in the 3500 lx group, compared to 1700 lx and dark groups. The production of carotenoids was promoted by the increase of light intensity. According to the results, the curve of the biomass growth did not display a synchronous trend with the production of carotenoids but the application of irradiation during cultivation could increase the yields of carotenoids biosynthesis in R. mucilaginosa.
Fig. 3.
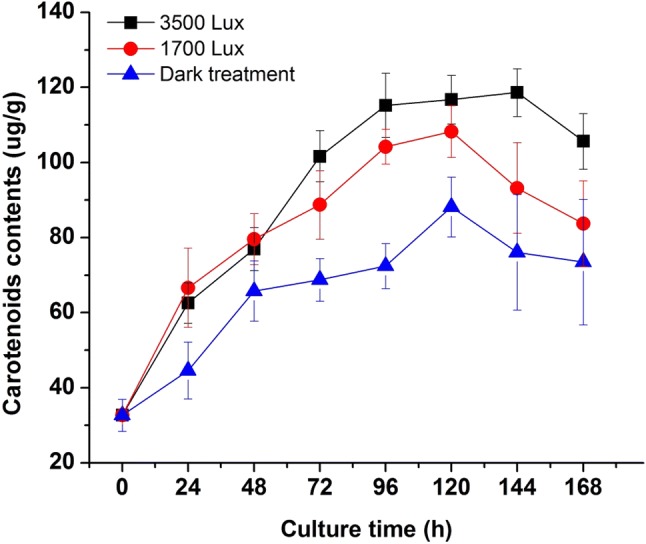
Effects of different light irradiation on the carotenoids production of R. mucilaginosa
Light is an essential element for the survival of a variety of microorganisms in nature, which can bring benefits to life as well as harm. High light intensity can produce singlet oxygen that can harm organisms. While carotenoids are natural pigments which are metabolized by a microorganism, play an important role in antioxidant activities (Skibsted 2012; Stephensen 2013). Light can produce reactive oxygen species (ROS) by photosensitizing with flavins or protein-bound flavins protein (Martin and Burch 1990). Carotenoids are important in protecting against photo-oxidative damage (Marova et al. 2012). Most heterotrophic yeast and bacteria rely on carotenoids for protection when growing in light (Yen and Zhang 2011; Yen and Yang 2012). The production of carotenoids is photo-dependent in some fungi, such as Phycomyces blakesleeanus, N. crassa. Or the carotenoids are synthesized during photomorphogenesis (Moore 1998). Therefore, oxygen stress under light culture condition is higher than that under dark culture condition (Georgiou et al. 2001). But more critically, some fungi (N. crassa, Mucor circinelloides) have carotenoids synthesis genes that depend on light-induced expression (Quiles-Rosillo et al. 2003). In our study, we observed the enhancement of carotenoids with the increase of light intensity, which implied photomorphogenesis and oxygen stress also existed in R. mucilaginosa under illumination and carotenoids biosynthesis could be a potential way in antioxidant function.
Effects of different light irradiation on lipids content and fatty acid compositions
The changes of lipids contents under different light irradiation were shown in Fig. 4. The results indicated that the group without irradiation had the highest lipid content among all trials, which might be inferred that radiation could enhance the growth of R. mucilaginosa, but the rapid cell growth rate reversely inhibited the accumulation of lipid. In the overall curve, the amount of lipids content without irradiation was higher than that of the other two groups. According to Fig. 4, in addition, it could be proved that light treatment did not improve the lipids content of R. mucilaginosa significantly (p < 0.05). Zhang et al. (2014) reported that dark/low-temperature could enhance lipid content, while irradiation/high-temperature increased the yields of biomass and carotenoid of R. glutinis. The total amounts of lipid production were very similar in three groups (without light, 2 and 3 LED) in spite of the lipid content decreased, that was due to the increase of biomass growth.
Fig. 4.
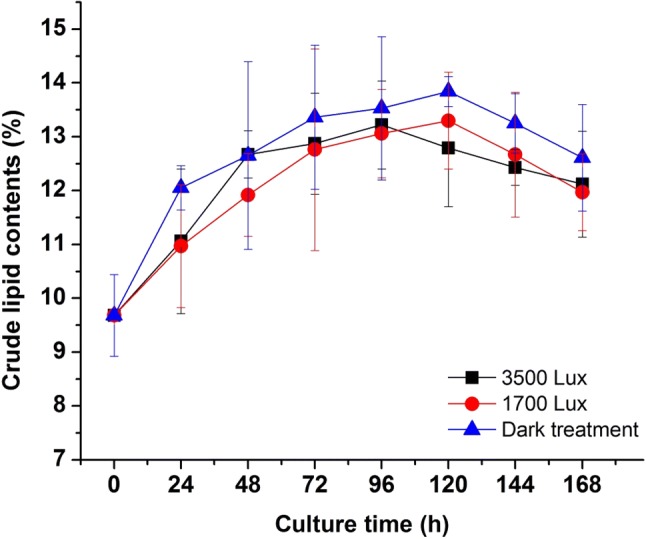
Effects of different light irradiation on the crude lipid contents of R. mucilaginosa
To further investigate the effects of light on the biosynthesis of fatty acid, the compositions of the crude lipids extracted from R. mucilaginosa were analyzed by GC–MS. As shown in Table 1, the main components of the fatty acid from R. mucilaginosa were octadecenoic acid C(18:1), hexadecanoic acid C(16:0), octadecanoic acid C(18:0), hexadecenoic acid C(16:1) and arachidic acid C(20:0). While, as the highest and major component, the percentage of octadecenoic acid was ranged from 72.24 to 75.12%. The compositions of fatty acid in this work were similar to the report by Perrier et al. (1995). Except for C(18:1) and C(16:0), the other fatty acid proportions in three trials displayed a certain significant difference. For instance, the proportion of C(16:0) in 3500 lx group was higher than the other two trails; meanwhile, the proportions of C(18:0) and C(20:0) in dark group were higher than the trails with illumination. Furthermore, the proportion of total saturated fatty acids (SFAs) was decreased from 18.66, 16.21 to 15.39% in dark, 1700 lx and 3500 lx group respectively. And the proportions of total monounsaturated fatty acids (C16:1 and C18:1) of dark group increased from 73.30 to 74.42% (1700 lx) and 77.29% (3500 lx) of the illumination trials, which indicated that light has an important effect on the synthesis of fatty acids in R. mucilaginosa.
Table 1.
Effects of different light irradiation on the fatty acid compositions of R. mucilaginosa (n = 3)
| Fatty acid compositions | Fatty acid content (%) | ||
|---|---|---|---|
| 0 lx | 1700 lx | 3500 lx | |
| Hexadecenoic acid/C(16:1) | 1.06 ± 0.03b | 1.10 ± 0.10b | 2.17 ± 0.38a |
| Hexadecanoic acid/C(16:0) | 8.79 ± 0.37a | 8.38 ± 0.25a | 8.24 ± 0.44a |
| Octadecenoic acid/C(18:1) | 72.24 ± 1.05a | 73.32 ± 0.04a | 75.12 ± 1.58a |
| Octadecanoic acid/C(18:0) | 8.61 ± 0.15a | 7.30 ± 0.07b | 6.67 ± 0.40b |
| Arachidic acid/C(20:0) | 1.26 ± 0.07a | 0.53 ± 0.06b | 0.48 ± 0.03b |
Data in table represent mean ± standard deviation; Lower letter represent (p < 0.05); Data followed by different lower letter are significantly different among treatments according to T test. Where (n = 3) is the number of days of culture
The effects of light on the synthesis of lipids and fatty acids in photosynthetic microalgae cells had been reported elsewhere, but few works in fungi. Light was one of the most important factors that microalgae cells changed the proportion of various lipids as the environment changes, thus changing the composition of fatty acid (Shi and Pan 2004). Early results showed that the increase in light intensity was beneficial to the formation of microalgae 16-carbon and 18-carbon polyunsaturated fatty acids (PUFAs). The previous work also showed that the PUFAs contents of C (18:2), C (20:4) and C (20:5) in Porphyridium cruentum increased with the increase of light intensity (Lee and Tan 1988). However, the study also showed that the proportion of microalgae unsaturated fatty acid (USFA) was decreased under high light intensity condition. Renaud et al. (1991) believed that it was due to the different types of desaturated enzymes exist in different microalgae cells, and the activity of these enzymes was affected by different light intensity. It had been reported that fatty acid dehydrogenase gene sequences exist in R. glutinis (Paul et al. 2014), moreover, Yang et al. (2015) also cloned and heterologous expressed of a Δ12-desaturase gene from R. glutinis. However, there were few works on photoinduction of desaturase gene about USFA biosynthesis in Rhodotorula. The data from Table 1 and Fig. 4 revealed that light irradiation could enhance the biosynthesis of USFA and reduce the proportion of SFAs, compared with the dark control. The results suggested that light stimulation probably activate the desaturase activity presented in R. mucilaginosa and promote the biosynthesis of USFA. Furthermore, regulation of the fatty acid compositions in R. mucilaginosa through adjusting light intensity might be a feasible approach to biosynthesis of functional USFA.
The changes of amino acids compositions under different light irradiation
Amino acid is the base of each biological protein synthesis. Therefore, the relative contents of amino acids in R. mucilaginosa cells with different irradiation were analyzed (Table 2). The results showed that in 18 amino acids, relative contents of 12 amino acids in different irradiation treatments showed a certain significant difference (p < 0.05), but except for threonine, serine, methionine, phenylalanine, cysteine, tryptophan. Compared with the dark group control, the relative contents of 12 amino acids including aspartic acid, glutamic acid, proline, glycine, alanine, valine, isoleucine, leucine, tyrosine, lysine, histidine, and arginine were decreased with irradiation. Such as, the relative content of glutamic acid in dark control was 1.68 times and 1.50 times in 1700 and 3500 lx groups, respectively. The total relative contents of amino acids in 1700 lx and 3500 lx were 20.79% and 23.54%, respectively, which were lower than the dark control of 28.97%. While the total protein content in 3500 lx group was higher than the group of 1700 lx illumination (p < 0.05). The data showed that light irradiation could regulate and reduce the biosynthesis of amino acids and proteins.
Table 2.
Effects of different light irradiation on the amino acid compositions of R. mucilaginosa (n = 3)
| Amino acid compositions | Amino acid contents (%) | ||
|---|---|---|---|
| 0 lx | 1700 lx | 3500 lx | |
| Aspartic acid | 2.37 ± 0.21a | 1.82 ± 0.12b | 1.96 ± 0.14b |
| Threonine | 1.26 ± 0.07a | 0.96 ± 0.14a | 1.03 ± 0.07a |
| Serine | 1.41 ± 0.21a | 1.09 ± 0.08a | 1.17 ± 0.09a |
| Glutamic acid | 4.49 ± 0.07a | 2.67 ± 0.15c | 2.99 ± 0.07b |
| Proline | 2.17 ± 0.13a | 0.66 ± 0.08c | 1.80 ± 0.08b |
| Glycine | 1.33 ± 0.13a | 1.06 ± 0.05b | 1.13 ± 0.06ab |
| Alanine | 1.93 ± 0.17a | 1.50 ± 0.04b | 1.60 ± 0.13ab |
| Valine | 2.11 ± 0.18a | 1.67 ± 0.05b | 1.76 ± 0.09b |
| Methionine | 1.29 ± 0.10a | 1.26 ± 0.11a | 1.33 ± 0.10a |
| Isoleucine | 1.12 ± 0.09a | 0.89 ± 0.07b | 0.97 ± 0.11ab |
| Leucine | 2.06 ± 0.07a | 1.58 ± 0.06b | 1.71 ± 0.08b |
| Tyrosine | 1.12 ± 0.11a | 0.93 ± 0.06b | 0.99 ± 0.07b |
| Phenylalanine | 1.25 ± 0.12a | 1.00 ± 0.15a | 1.07 ± 0.07a |
| Lysine | 1.71 ± 0.06a | 1.17 ± 0.04c | 1.36 ± 0.06b |
| Histidine | 0.62 ± 0.05a | 0.46 ± 0.06b | 0.50 ± 0.06ab |
| Arginine | 2.09 ± 0.07a | 1.48 ± 0.09b | 1.54 ± 0.11b |
| Cysteine | 0.19 ± 0.05a | 0.22 ± 0.04a | 0.22 ± 0.07a |
| Tryptophan | 0.43 ± 0.09a | 0.34 ± 0.07a | 0.42 ± 0.04a |
| Total | 28.97 ± 0.63a | 20.79 ± 1.11c | 23.54 ± 0.94b |
Date in table represent mean ± standard deviation; Lower letter represent 0.05 level; Date followed by different lower letter are significantly different within a column according to T test. The tested samples were dried solid cell powder, the values are relative percentage (%)
There were few reports related to the effects of light irradiation on the biosynthesis of proteins and amino acids in microorganisms. Previous study reported that the relative concentrations of secreted proteins in liquid cultures of N. crassa differed in constant darkness compared to constant light (2500 lx). Light reduced the concentrations of some polypeptides markedly and increased the concentrations of protein species of 67, 40, 18 and 13 kDa (Kallies et al. 1992). The changes of amino acid compositions under dark and illumination groups might be attributed to the part of amino acids and proteins participated in the cytoprotection response to photooxidation (Kerwin and Remmele 2007). The molecular regulation mechanism of light on protein and amino acid synthesis in R. mucilaginosa has not been reported, which is a question worthy of further study.
Conclusion
The results showed that different light intensities influence on the growth kinetics and biochemical components of R. mucilaginosa compared to the dark control. Appropriate irradiation could promote growth and carotenoids production of the pigment-producing fungus obviously, stimulated the biosynthesis of USFAs while decreases the amino acids and total protein contents. Moreover, strong illumination inhibited the growth of the cells and glucose consumption. Our findings concluded that light irradiation could affect the growth characteristics and metabolites compositions of R. mucilaginosa, which suggested that photo-regulatory factors might exist in non-photosynthetic fungi that could synthesize carotenoids. Moreover, light regulation may be a feasible way to control the biosynthesis and production of bioactive ingredients in the pigmented microorganism.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 31360192), and Team Project of Longyuan Youth Innovation & Entrepreneurship (2018).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Weibao Kong, Phone: +86 931-7971912, Email: kwbao@163.com.
Shiquan Niu, Email: sqniu@nwnu.edu.cn.
References
- Andrade MR, Costa JAV. Mixotrophic cultivation of microalga Spirulina platensis using molasses as organic substrate. Aquaculture. 2007;264:130–134. [Google Scholar]
- Babitha S, Carvahlo JC, Soccol CR, Pandey A. Effect of light on growth, pigment production and culture morphology of Monascus purpureus in solid-state fermentation. World J Microbiol Biotechnol. 2008;24:2671–2675. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Buzzini P, Martini A. Production of carotenoids by strains of Rhodotorula glutinis, cultured in raw materials of agro-industrial origin. Bioresour Technol. 2000;71:41–44. [Google Scholar]
- Corrochano LM. Fungal photoreceptors: sensory molecules for fungal development and behaviour. Photochem Photobiol Sci. 2007;6:725–736. doi: 10.1039/b702155k. [DOI] [PubMed] [Google Scholar]
- Dasgupta A, Fuller KK, Dunlap JC, Loros JJ. Seeing the world differently: variability in the photosensory mechanisms of two model fungi. Environ Microbiol. 2015;18:5–20. doi: 10.1111/1462-2920.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Determination of amino acids in food, GB 5009.124-2016, Chinese National Standard
- Dworecka-Kaszak B, Kizerwetter-Swida M. Pseudomycelium forming Rhodotorula: unusual picture of biofilm. Mikologia Lekarska. 2011;18:74–78. [Google Scholar]
- Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fu MJ, Zou ZR. Formation of pycnidium and accumulation of carotenoid in Pestalotiopsis sp. F induced by blue light. Food Sci. 2009;30:118–121. [Google Scholar]
- Georgiou CD, Zervoudakis G, Tairis N, Kornaros M. β-carotene production and its role in sclerotial differentiation of Sclerotium rolfsii. Fungal Genet Biol. 2001;34:11–20. doi: 10.1006/fgbi.2001.1285. [DOI] [PubMed] [Google Scholar]
- Hernández-Almanza A, Montanez JC, Aguilar-González MA, Martínez-Ávila C, Rodríguez-Herrera R, Aguilar CN. Rhodotorula glutinis, as source of pigments and metabolites for food industry. Food Biosci. 2014;5:64–72. [Google Scholar]
- Kallies A, Mohsenzadeh S, Rensing L. Effects of light on protein secretion in Neurospora crassa. Arch Microbiol. 1992;157:104–106. doi: 10.1007/BF00245276. [DOI] [PubMed] [Google Scholar]
- Kerwin BA, Jr, Remmele RL. Protect from light: photodegradation and protein biologics. J Pharm Sci. 2007;96:1468–1479. doi: 10.1002/jps.20815. [DOI] [PubMed] [Google Scholar]
- Kong WB, Yang H, Cao YT, Song H, Hua SF, Xia CG. Effect of glycerol and glucose on the enhancement of biomass, lipid and soluble carbohydrate production by Chlorella vulgaris in mixotrophic culture. Food Technol Biotechnol. 2013;51:62–69. [Google Scholar]
- Lee YK, Tan HM. Effect of temperature, light intensity and dilution rate on the cellular composition of red alga Porphyridium cruentum, in light-limited chemostat cultures. World J Microbiol Biotechnol. 1988;4:231–237. [Google Scholar]
- Llorente B, Martinez-Garcia JF, Stange C, Rodriguez-Concepcion M. Illuminating colors: regulation of carotenoid biosynthesis and accumulation by light. Curr Opin Plant Biol. 2017;37:49–55. doi: 10.1016/j.pbi.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Malisorn C, Suntornsuk W. Improved β-carotene production of Rhodotorula glutinis in fermented radish brine by continuous cultivation. Biochem Eng J. 2009;43:27–32. [Google Scholar]
- Marova I, Carnecka M, Halienova A, Certik M, Dvorakova T, Haronikova A. Use of several waste substrates for carotenoid-rich yeast biomass production. J Environ Manag. 2012;95:338–342. doi: 10.1016/j.jenvman.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Martin JP, Jr, Burch P. Production of oxygen radicals by photosensitization. Methods Enzymol. 1990;186:635–645. doi: 10.1016/0076-6879(90)86159-s. [DOI] [PubMed] [Google Scholar]
- Method for determination of fatty acid methyl-ester from animal and plant lipids, GBT17376-2008, Chinese National Standard
- Method for determination of tryptophan in feed—spectrophotometry. GB/T 15400-1994, Chinese National Standard
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Biochem. 1959;31:426–428. [Google Scholar]
- Moore D. Fungal morphogenesis. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Morelli G, Nelson MA, Ballario P, Macino G. Photoregulated carotenoid biosynthetic genes of Neurospora crassa. Methods Enzymol. 1993;214:412–424. doi: 10.1016/0076-6879(93)14085-w. [DOI] [PubMed] [Google Scholar]
- Olmedo M, Ruger-Herreros C, Luque EM, Corrochano LM. Regulation of transcription by light in Neurospora crassa: a model for fungal photobiology? Fungal Biol Rev. 2013;27:10–18. [Google Scholar]
- Olmedo M, Roenneberg T, Merrow M, Corrochano LM. Glucose sensing and light regulation: a mutation in the glucose sensor RCO-3 modifies photoadaptation in Neurospora crassa. Fungal Biol. 2018;122:497–504. doi: 10.1016/j.funbio.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Park YK, Nicaud JM, Ledesma-Amaro R. The engineering potential of Rhodosporidium toruloides as a workhorse for biotechnological applications. Trends Biotechnol. 2018;36:304–317. doi: 10.1016/j.tibtech.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Paul D, Magbanua Z, Arick M, French T, Bridges SM, Burgess SC, Lawrence ML. Genome sequence of the oleaginous yeast Rhodotorula glutinis ATCC 204091. Genome Announc. 2014;2:e00046-14. doi: 10.1128/genomeA.00046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier V, Dubreucq E, Galzy P. Fatty acid and carotenoid composition of Rhodotorula strains. Arch Microbiol. 1995;164:173–179. doi: 10.1007/BF02529968. [DOI] [PubMed] [Google Scholar]
- Quiles-Rosillo MD, Torres-Martínez S, Garre V. cigA, a light-inducible gene involved in vegetative growth in Mucor circinelloides is regulated by the carotenogenic repressor crgA. Fungal Genet Biol. 2003;38:122–132. doi: 10.1016/s1087-1845(02)00519-4. [DOI] [PubMed] [Google Scholar]
- Renaud SM, Parry DL, Thinh LV, Kuo C, Padovan A, Sammy N. Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis, sp. and Nannochloropsis oculata, for use in tropical aquaculture. J Appl Phycol. 1991;3:43–53. [Google Scholar]
- Rodriguez-Romero J, Hedtke M, Kastner C, Müller S, Fischer R. Fungi, hidden in soil or up in the air: light makes a difference. Annu Rev Microbiol. 2010;64:585–610. doi: 10.1146/annurev.micro.112408.134000. [DOI] [PubMed] [Google Scholar]
- Rua J, Rodriguezaparicio LB, Busto F, Soler J. Effect of light on several metabolites of carbohydrate metabolism in Phycomyces blakesleeanus. J Bacteriol. 1987;169:904–907. doi: 10.1128/jb.169.2.904-907.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenge C, Cheirsilp B, Bourtoom T. Efficient concomitant production of lipids and carotenoids by oleaginous red yeast Rhodotorula glutinis cultured in palm oil mill effluent and application of lipids for biodiesel production. Biotechnol Bioprocess Eng. 2011;16:23–33. [Google Scholar]
- Sakaki H, Nakanishi T, Tada A, Miki W, Komemushi S. Activation of torularhodin production by Rhodotorula glutinis using weak white light irradiation. J Biosci Bioeng. 2001;92:294–297. doi: 10.1263/jbb.92.294. [DOI] [PubMed] [Google Scholar]
- Schreiber M, Petersen H, Wachsmuth C, Müller H, Hufert FT, Schmitz H. Mechanism of photoregulated carotenogenesis in Rhodotorula minuta I. photocontrol of carotenoid production. Plant Cell Physiol. 1982;23:541–547. [Google Scholar]
- Shi J, Pan K. Effects of different culture conditions and growth phases on lipid of microalgae. Mar Fish Res. 2004;25:79–85. [Google Scholar]
- Skibsted LH. Carotenoids in antioxidant networks. Colorants or radical scavengers. J Agric Food Chem. 2012;60:2409–2417. doi: 10.1021/jf2051416. [DOI] [PubMed] [Google Scholar]
- Stephensen CB. Provitamin A carotenoids and immune function. Carotenoids and human health. Totowa: Humana Press; 2013. pp. 261–270. [Google Scholar]
- Tada M, Shiroishi M. Mechanism of photoregulated carotenogenesis in Rhodotorula minuta II. Aspects of photoregulative reaction. Plant Cell Physiol. 1982;23:549–556. [Google Scholar]
- Wang C, Yang H, Chen M, Wang Y, Li F, Luo C, Zhao S, He D. Real-time quantitative analysis of the influence of blue light on citrinin biosynthetic gene cluster expression in Monascus. Biotechnol Lett. 2012;34:1745–1748. doi: 10.1007/s10529-012-0962-z. [DOI] [PubMed] [Google Scholar]
- Yang X, He S, Wei Y, Lin L, Ji X, Zhang Q. Cloning and heterologous expression of a Δ12-desaturase gene from Rhodotorula glutinis. Chin J Appl Environ Biol. 2015;21:421–426. [Google Scholar]
- Yen HW, Yang YC. The effects of irradiation and microfiltration on the cells growing and total lipids production in the cultivation of Rhodotorula glutinis. Bioresour Technol. 2012;107:539–541. doi: 10.1016/j.biortech.2011.12.134. [DOI] [PubMed] [Google Scholar]
- Yen HW, Zhang Z. Enhancement of cell growth rate by light irradiation in the cultivation of Rhodotorula glutinis. Bioresour Technol. 2011;102:9279–9281. doi: 10.1016/j.biortech.2011.06.062. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang X, Tan T. Lipid and carotenoid production by Rhodotorula glutinis under irradiation/high-temperature and dark/low-temperature cultivation. Bioresour Technol. 2014;157:149–153. doi: 10.1016/j.biortech.2014.01.039. [DOI] [PubMed] [Google Scholar]


