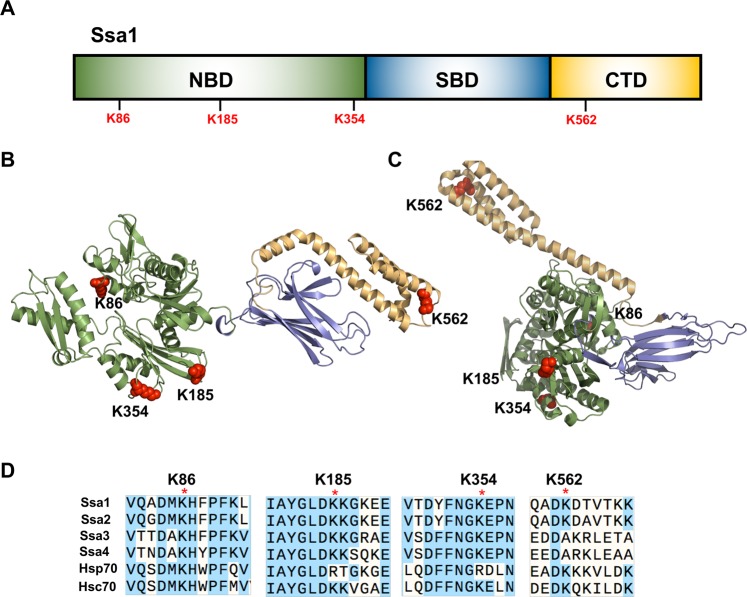
Figure 1.
Heat shock alters acetylation of Ssa1. (A) Domain structure of Ssa1. All lysine residues that were found to be deacetylated upon heat shock as deretmined by mass spectrometry are indicated. (B,C) Cartoon representation of Hsp70 in the ADP-bound open conformation (PDB: 2KHO) and in the ATP-bound closed conformation (PDB: 4JNE) showing the NBD (green), SBD (blue) and CTD lid (yellow). The four deacetylated residues are highlighted in red. (D) Conservation of the deacetylated residues in Hsp70. amino acid sequences of Saccharomyces cerevisiae Hsp70 isoforms Ssa1, Ssa2, Ssa3 and Ssa4 as well as sequences for human Hsc70 and Hsp70 were aligned using Snapgene. Amino acids identified as becoming deacetylated upon heat shock are annotated with a red dot. Raw mass spectrometry data are available via ProteomeXchange with identifier PXD015185.