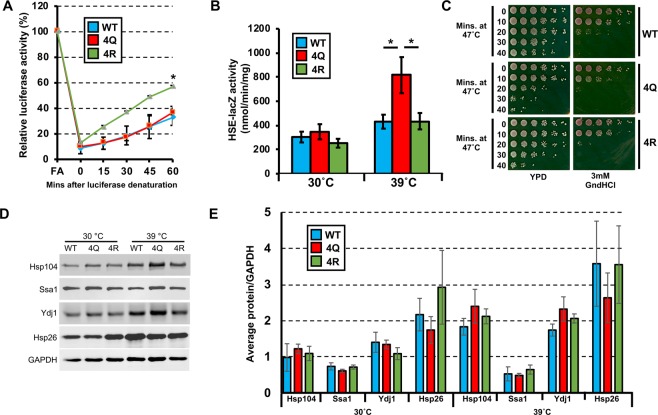
Figure 3.
Effects of Ssa1 deacetylation on in vivo function. (A) Ability of Ssa1 (WT, 4Q and 4R) to refold Luciferase over a 60 min time course. Activity relative to fully active luciferase was calculated and data shown are the average and SD of three independent experiments. (B) Effects of acetylation of Ssa1 on HSF1 activation. A plasmid containing the HSE-lacZ reporter gene was transformed into G402 cells. Cells were cultured at 30 °C or heat shock at 39 °C for 2 h. The activation of Hsf1 was calculated by measuring the expression of β-galactosidase under Heat Shock Element (HSE) control. Data are the average and SD of three independent experiments. *Represents p < 0.05. (C) Acquired thermotolerance assay of acetylation site mutants. Fresh cultures were pre-treated at 39 °C for 1 h, then cells were heat shocked at 47 °C for the indicated times and then plated on media either containing or lacking 3 mM Gdn-HCl (an inhibitor of Hsp104). (D) Steady state levels of major co-chaperone proteins in acetylation site mutants. WT, 4Q and 4R cells were grown to exponential phase and were either incubated at 30 °C or 39 °C for 2 hours. Cell extracts were obtained, resolved on SDS-PAGE gels and analyzed by immunoblotting with anti-Hsp104, Ssa1, Ydj1 and Hsp26 antibodies. GAPDH was used as a loading control. (E) Quantitation of major co-chaperone in acetylation site mutants. Data shown are the average and SD of three independent experiments.