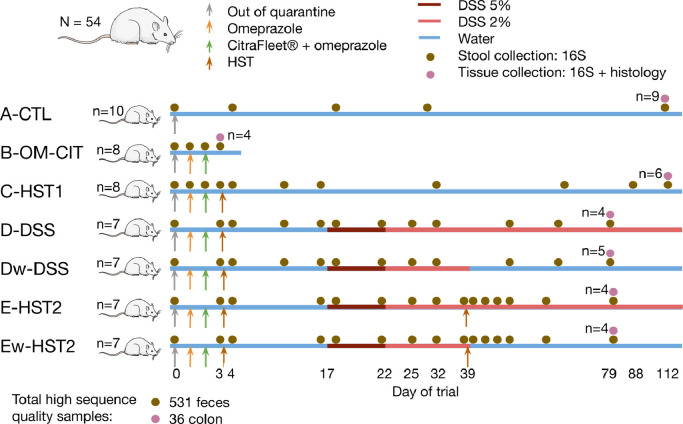
Fig. 2.
Experimental design to evaluate the effect of a human stool transfer on a rat model of chronic intestinal inflammation. Seven groups were distributed as follows: a control group receiving a standard diet and water without treatment (group A-CTL, n = 10); a group receiving omeprazole and Citrafleet® (group B-OME-CIT, n = 8); a group receiving omeprazole, Citrafleet® and a human stool (group C—HST1, n = 8); a group receiving omeprazole, Citrafleet®, human stool, and DSS until day 39, which was replaced by water (w) until the end of the study (group Dw-DSS, n = 7); a group receiving omeprazole, Citrafleet®, human stool and then DSS until the end of the study (group d-DSS, n = 7); a group receiving omeprazole, Citrafleet®, human stool, and DSS until day 39, which was replaced by water (w) until the end of the study, and a second human stool (group Ew-HST2, n = 7); a group receiving omeprazole, Citrafleet®, human stool, DSS until the end of the study, and a second human stool (group E-HST2, n = 7). HST: Human stool transfer; DSS: dextran sodium sulfate. The “n” in the right side of the figure represents the number of animals for which colonic tissues were collected and analysed for microbiome composition.