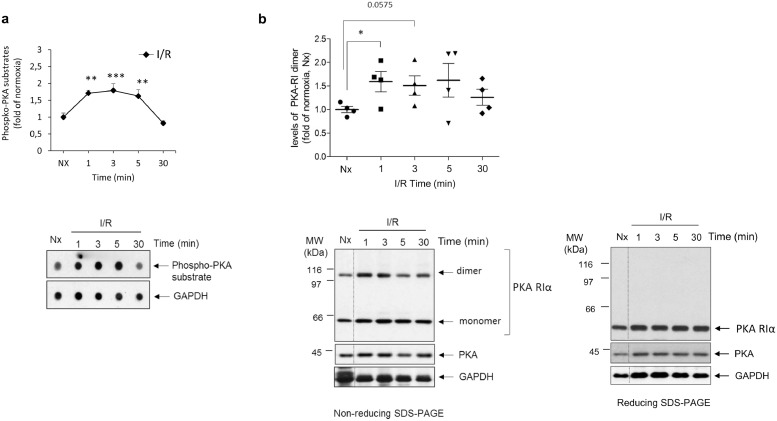
Fig. 2.
Cardiac ischemia/reperfusion induces rapid and transient oxidation-dependent activation of PKA. (A) Global PKA activity was assessed in lysates from normoxic or reperfused isolated rat hearts (40 min of sustained ischemia followed by the indicated reperfusion times) by dot-blot, using a pan-specific phospho-substrate antibody that broadly detects phosphorylated proteins by PKA as indicated in Methods. GAPDH expression was used as loading control. Data are mean ± SEM, n = 3–4 rats per condition. **p < .01; ***p < .001 compared to I/R 30 min [1-way ANOVA and Tukey's post hoc test]. A representative dot-blot is shown. (B) I/R promotes oxidation-related dimerization of the regulatory PKA-RIα subunit in parallel to global PKA activation. Lysates as in panel A were probed with a specific PKA-RI antibody that recognizes both monomers and dimers of this regulatory subunit linked by disulfide bond formation. The dimer formation detected in non-reducing SDS-PAGE resolving conditions was fully reversed in the presence of 2-mercaptoethanol (reducing SDS-PAGE). The expression of the PKA catalytic subunit and of GAPDH was used as loading controls. Data are mean ± SEM, n = 3–4 rats per condition. *p < .05 for the indicated comparisons [Student's t-test]. Representative blots are shown.