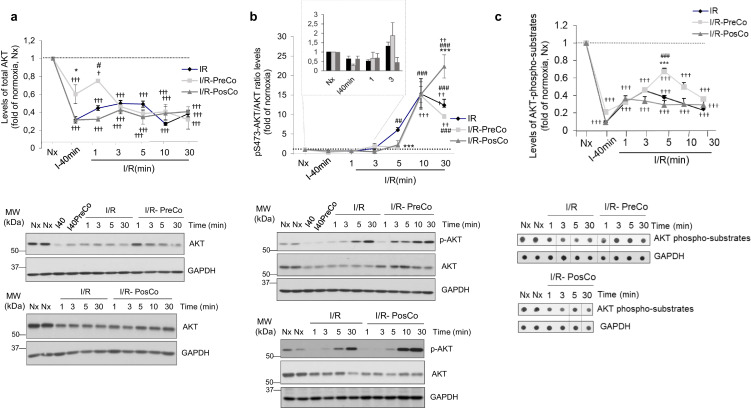
Fig. 4.
Ischemia/reperfusion promotes a marked loss of total AKT protein and an overall decrease in global AKT-mediated substrate phosphorylation, despite the robust stimulation status of the remaining AKT protein. Rat heart lysates as in Fig. 1 were analyzed with specific antibodies for total AKT (A) or for AKT phosphorylated at Ser473 (B) as detailed in Methods. Data were normalized by GAPDH (panel A) or total AKT (panel B) loading. (C) Global activity of AKT towards its substrates was assessed in cardiac lysates from the indicated conditions by dot-blot, using a pan phospho-AKT substrate-specific antibody, and data normalized by GAPDH loading. In all panels, normalized data were represented as fold-change with respect to normoxic situation and are mean ± SEM, n = 3–4 rats per condition. Data were analyzed by comparing the different experimental situations (I/R, I/R-PreCo and I/R-PosCo) to the normoxic condition (2-way ANOVA followed by Bonferroni's post-hoc test, †p < .05; ††p < .01, †††p < .001). In addition, we compared I/R pre-Co and I/R post-Co conditions versus I/R alone (*p < .05; ***p < .001) or between conditioning situations (#p < .05; ##p < .01, ###p < .001) [1-way ANOVA and Tukey's post hoc test]. Representative blots are shown.