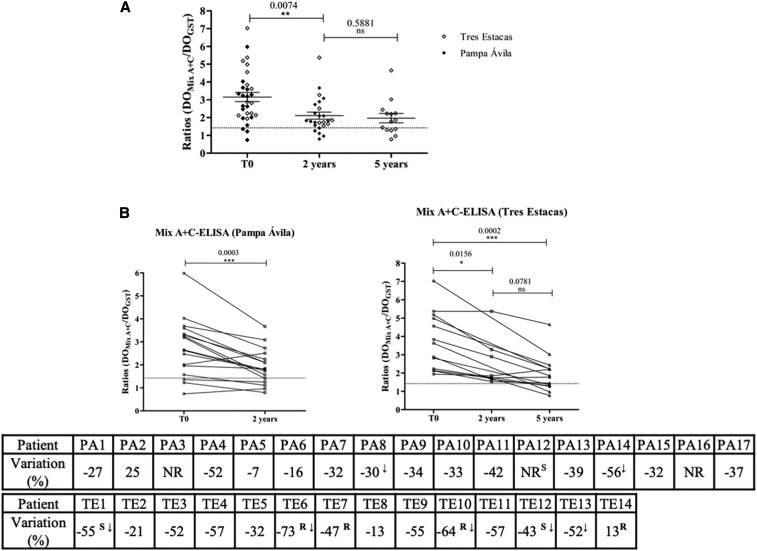
Figure 2.
Follow-up of treated children using Mix A + C–ELISA. Patients from rural settlements “Tres Estacas” and “Pampa Ávila,” Chacabuco Department, province of Chaco, were included in this study. In “Pampa Ávila” blood samples were collected before treatment (T0; 2007) and 2 years after treatment (T1; 2009); in Tres Estacas, blood samples were taken before treatment (T0; 2004) and 2 and 5 years after treatment (T1; 2006 and T2; 2009). (A) Pre- and posttreatment reactivity against Mix A + C–ELISA of all treated children. (B) Individual follow-up of anti-TcTASV antibody levels after treatment in each settlement. The degree of variation (%) was calculated as: ([Final Abs-Initial Abs]/Initial Abs) × 100.19 A negative sign in the percentage indicates a decrease in posttreatment antibody levels, whereas a positive sign indicated an increase. ↓ = patients who seroconverted. A decrease greater than 30% with respect to T0 is considered a positive therapy impact9; a decrease greater than 75% in antibody levels is indicative of a tendency to seroconversion.19 Differences between pretreatment and posttreatment reactivity were evaluated by the paired comparison Wilcoxon test (P < 0.05). NR = indicates sera that were not reactive by Mix A + C–ELISA at T0. R = sera that showed a significative reduction in titers or S = seroconverted by ELISA, as previously evaluated by Monje-Rumi et al.17