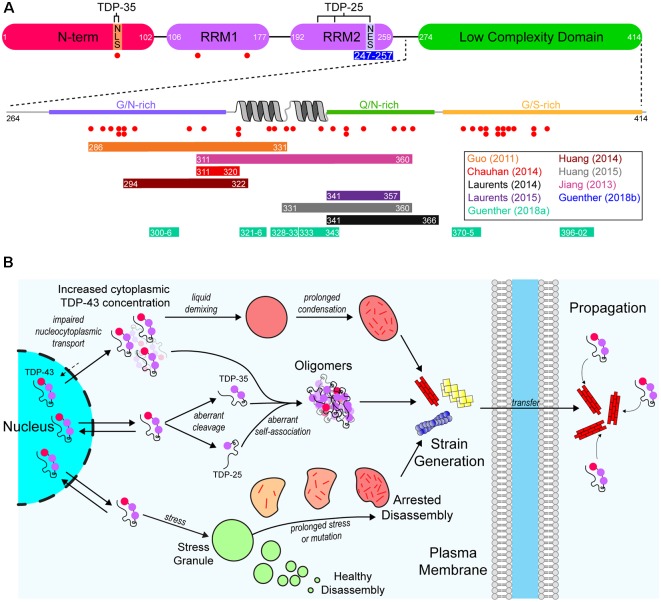
Figure 2.
A model of the aggregation and prion-like propagation of TAR DNA-binding protein of 43 kDa (TDP-43) in ALS. (A) TDP-43 is composed of four domains which include the ubiquitin-like N-terminal domain (NTD), tandem RNA-recognition motifs (RRM), and a C-terminal low complexity domain (LCD). The NTD contains the nuclear localization signal (NLS) of TDP-43, whereas the nuclear export signal (NES) is in RRM2. The pathological 35 kDa (TDP-35) and 25 kDa (TDP-25) are formed via aberrant caspase-mediated cleavage at the NLS and in RRM2 respectively. The blue bar below RRM2 represents an amyloidogenic segment. The LCD is a prion-like domain due to it being enriched for G/N/Q/S residues. It also contains a helix-turn-helix motif which is important for association with other TDP-43 LCDs. Red dots below both the main TDP-43 graphic and the LCD expansion represent ALS-associated mutations. Colored bars represent experimentally identified regions of the protein that are capable of forming amyloid-like structures. Bar colors correspond to the publications listed on the right. (B) TDP-43 is a primarily nuclear-localized protein, however, it shuttles between the nucleus and cytoplasm. Impairment of nucleocytoplasmic transport (top) can lead to the cytoplasmic concentration of TDP-43 increasing, leading to liquid demixing to form biomolecular condensates distinct from stress granules. Prolonged residency of TDP-43 in these condensates can lead to the formation of aggregates. Aberrant cleavage (middle) leads to the formation of the highly aggregation-prone TDP-35 and TDP-25 pathological fragments. These fragments, and even full-length TDP-43 can aberrantly self-associate to form oligomers that may grow to become larger aggregates. Also, TDP-43 is known to inhabit stress granules (bottom). Given prolonged stress or genetic mutations, stress granules can persist for longer than necessary and can fail to disassemble, which leads to the formation of aggregates. Each of these possible pathways may also result in strains of TDP-43 aggregates that can propagate from cell-to-cell and seed further aggregation.