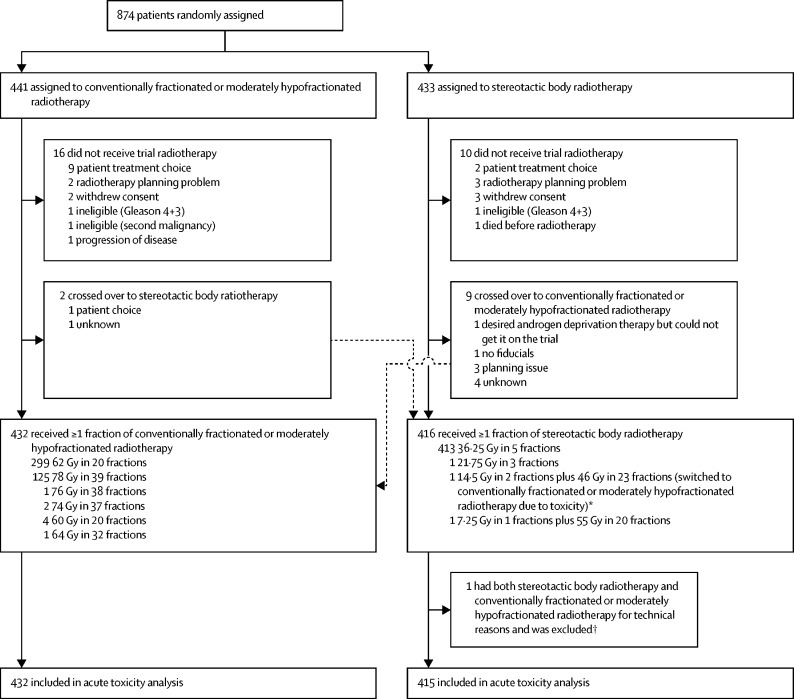
Figure 1.
Trial profile
Crossovers between treatment groups were analysed per-protocol for this acute toxicity substudy. Dose fractionation regimens administered within each group are shown. *One patient who received two fractions of stereotactic body radiotherapy then developed grade 3 toxicity (urosepsis) and switched to conventionally fractionated or moderately hypofractionated radiotherapy (further 46 Gy in 23 fractions) was not excluded from the toxicity analysis because he had toxic effects after two fractions of sterotactic body radiotherapy. †One patient who received a single incomplete fraction of stereotactic body radiotherapy (<7·25 Gy, set-up issues) and switched to conventionally fractionated or moderately hypofractionated radiotherapy (further 55 Gy in 20 fractions) was excluded.