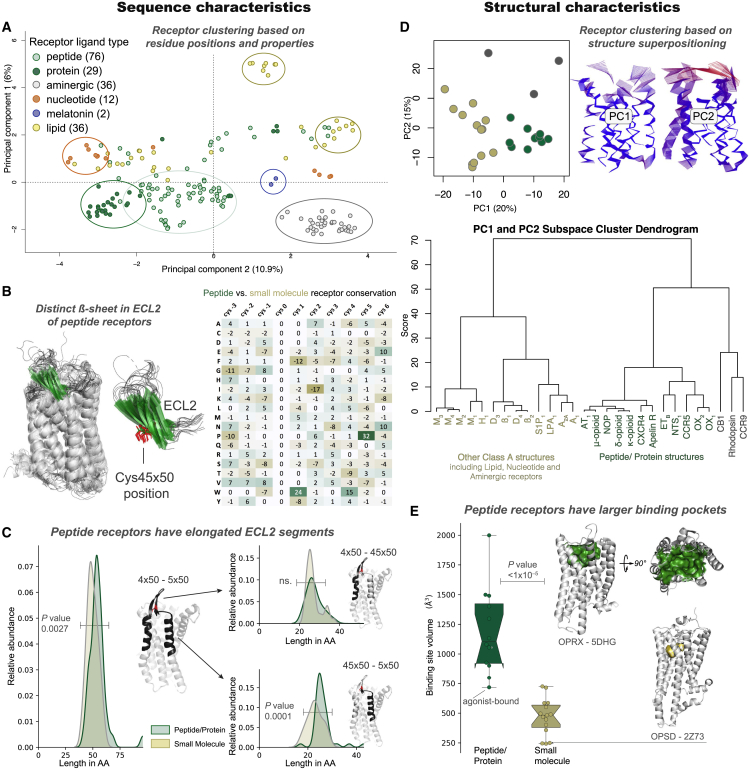
Figure 3.
Peptide Receptors Share Distinct Sequence and Structural Characteristics
(A) The majority of class A GPCRs cluster by endogenous ligand type based on ligand-interacting residue analysis with multi-dimensional scaling.
(B) Peptide receptors with a structure (n = 21, left) share a characteristic β sheet (green) substructure (left) and sequence (right) in extracellular loop 2 (ECL2), which includes a conserved cysteine, Cys45×50 (red, center).
(C) A long ECL2 segment (>20 residues) after Cys45×50 is an overrepresented feature of peptide/protein receptors (Wilcoxon rank-sum test, p < 1 × 10−5).
(D) Principal-component analysis of receptor structures in a 2D plot (top left) and dendrogram (bottom) demonstrate separation of peptide (green), non-peptide receptors (beige), and outliers (gray). Differences are predominantly found in the extracellularly facing ligand-binding domain, as shown by residue displacements from the mean (right).
(E) Ligand-binding pocket volumes are larger in peptide than non-peptide class A receptors.
See also Table S3 for related 3D PCA and ECL2 motif data.