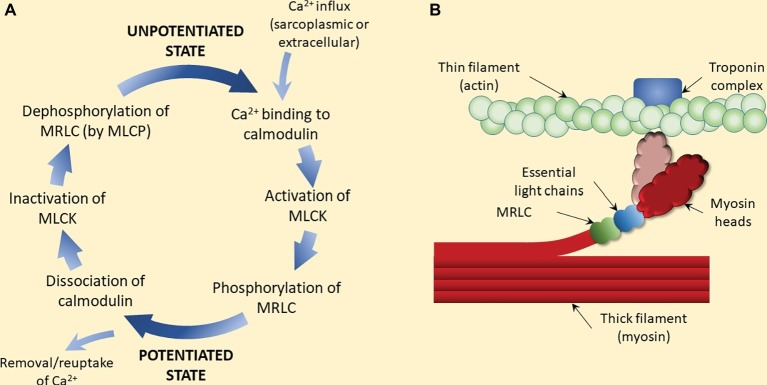
Figure 3.
Proposed mechanism of (classic) post-activation potentiation (PAP). (A) Calcium ions activate myosin light chain kinase (MLCK) subsequent to calcium-calmodulin interaction. MLCK then phosphorylates the myosin regulatory light chain (MRLC), which is believed to trigger the rotation of the myosin head away from the thick filament (myosin backbone) toward the thin filament (light color myosin head in panel B). Removal or reuptake of calcium triggers the dissociation of calmodulin and inactivation of MLCK. Dephosphorylation of the MRLC by myosin light chain phosphatase (MLCP) completes the process of potentiation removal. (B) MRLC phosphorylation and subsequent rotation of the myosin head (light color) increases the probability of the head attaching to actin, and thus force production. At submaximal (i.e., below saturating) levels of calcium, this process increases force output at a given calcium concentration, i.e., calcium sensitivity. Since phosphorylation is rapid but dephosphorylation is relatively slow, a period of contractile activity (e.g., a warm-up period) is sufficient to augment contractile properties in subsequent contractions, even if a short period of rest is imposed (e.g., contractions are intermittent) (Manning and Stull, 1979).