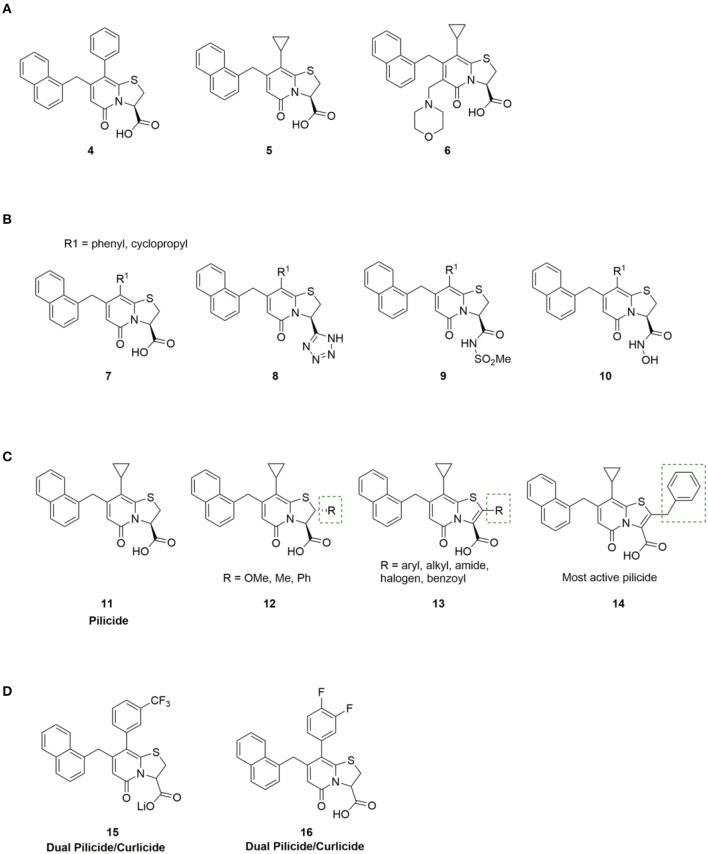
Figure 3.
(A) First generation pilicides, compound 4 and 5 (Svensson et al., 2001; Lee, 2003) suffered from low solubility. Aminomethylene substitution of pilicide 5 resulted in compound 6 with improved solubility properties (Hedenstrom et al., 2005). (B) Pilicides containing carboxylic acid isosteres, including tetrazoles (8), acyl sulfonamides (9) and hydroxamic acids (10) are tolerated (Åberg et al., 2008). (C) SAR study to investigate substitution of position C-2 in the pilicide scaffold, including both saturated (12) and unsaturated (13) analogs (Chorell et al., 2010). (D) Small modifications in the phenyl ring allowed identification of ring-fused 2-pyridones that exhibit dual pilicide-curlicide activity (Cegelski et al., 2009).