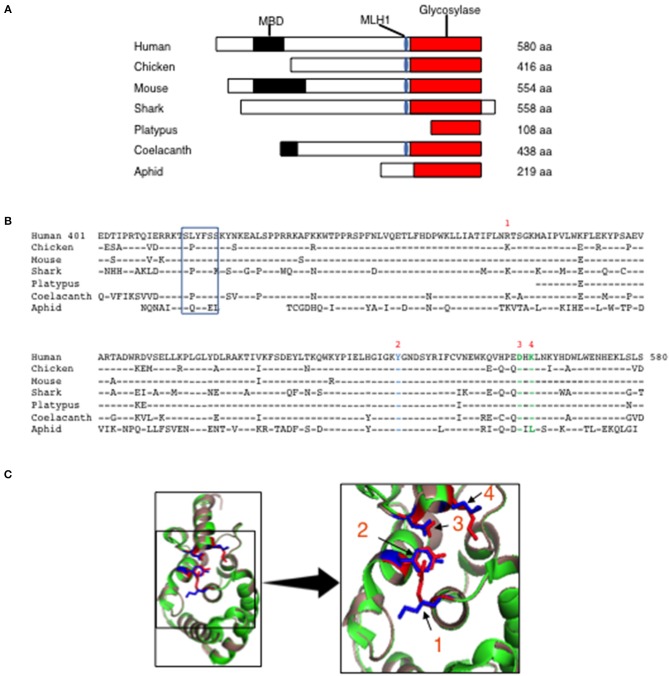
Figure 1.
Evolutionary conservation of MBD4 glycosylase domain. (A) Schematic (drawn to scale) of MBD4 protein in human (Homo sapiens), chicken (Gallus gallus), mouse (Mus musculus), ghost shark (Callorhinchus milii), platypus (Ornithorhynchus anatinus), ocean coelacanth (Latimeria chalumnae), and green aphid (Schizaphis graminum). The methyl binding domain (MBD) (black box), MLH1 binding domain (blue oval) and glycosylase domain (red box) are indicated as appropriate and amino acid (aa) number is listed. (B) The annotated human glycosylase domain exons 4–8 (aa 401–580) was used as the original sequence. Multiple aa alignments were performed showing conservation with chicken aa 237-416 (94.4%), mouse aa 375–554 (93.3%), ghost shark aa 347–528 (87.2%), platypus aa 1–108 (92.6%), ocean coelacanth aa 259–438 (90.6%) and green aphid aa 59–219 (70.4%). Conserved aa are indicated by dashes and non-conserved aa are noted using the single letter aa code. The MLH1 binding domain is boxed and the predicted catalytic amino acids in the glycosylase domain are numbered R368 (1), Y540 (2), D560 (3) and K562 (4) using the human MBD4 aa numbering system. (C) (Left panel) The three-dimensional (3D) crystal structure of MBD4 glycosylase domain from human (green) (27) and the predicted chicken (dark salmon) structure were aligned using PyMOL with catalytic amino acids shown in red (human) and blue (chicken). (Right panel) Comparison of the crystal structure involving the catalytic aa, R368 (1), Y540 (2), D560 (3), and K562 (4) in the human (red) and chicken (blue) glycosylase domains is shown in high magnification.