Abstract
Background
In the mid-1970s, an unusual chronic kidney disease of multifactorial origin (CKDmfo), also known as CKD of unknown aetiology (CKDu), began to manifest in several economically poor, tropical, agricultural countries. This preventable, environmentally induced, occupational disease affects several peri-equatorial countries; it first manifested in Sri Lanka in the mid-1990s. The study goal was to estimate the costs of eradicating CKDmfo and the resulting cost savings, using CKDmfo in Sri Lanka as an example. This chronic disease model is applicable to CKDu and few other chronic diseases in other countries.
Methodology
Eight cost-effective, key interventions were identified that are essential to eradicate CKDmfo. A systematic assessment was performed on these interventions (including providing clean water, behavioural and lifestyle changes, alleviating malnutrition, reducing irresponsible and overuse of agrochemicals, and cost-effective treatment options), the cost of prevention, and the resultant cost savings. A cost–benefit analysis was based on the data collected during the past 20 years of work in Sri Lanka.
Findings
The yearly cost required to eradicate the disease was approximately one-tenth of the current annual operating and opportunity costs due to CKDmfo. Analysis indicates that implementation of a focussed chronic disease-prevention plan using essential multiple interventions, CKDmfo can be eradicated within 15 years. This includes provision of potable water; real-time disease surveillance program; public and professional education; prevention of environmental pollution; alleviation of poverty and associated malnutrition; sustainable self-sufficiency in food, clean water, energy, and security; diversification of economy and job opportunities; sustainable economic development; regionwide programs of effective screening, early diagnosis and intervention to reverse the disease progression at earliest possible; and effective treatment of CKDmfo.
Interpretation
This analysis is based on multiple population-level, chronic disease-eradication strategies that include an interdisciplinary, geographic information system (GIS)-based, regionwide, long-term research and intervention program; economic diversification; and environmental, socioeconomic, and behavioural improvements. Such an approach will facilitate identification of root causes and key risk factors, enabling implementation of cost-effective longer-term interventions to eradicate chronic diseases, applicable to other countries as well.
Keywords: Economics, Health profession, Epidemiology, Internal medicine, Pathology, Physiology, Public health, Economic analysis, Pollution, Sri Lanka, CKDu, Socioeconomic, Soil, Agriculture, Premature deaths, CKDmfo, Cost-effective
1. Introduction
1.1. Chronic kidney disease of multifactorial origin (or unknown origin)
Chronic kidney disease of multifactorial origin (CKDmfo), also known as CKD of unknown origin (CKDu, CKDuo), is a serious public health problem predominantly affecting tropical countries [1, 2, 3]. This fatal, non-communicable disease predominantly affects middle-age male farmers [4]. No convincing evidence exists that the disease is caused by heavy metals, arsenic, agrochemicals, or microbes; spread from person to person (i.e., contagiouse), or have predominant genetic factors or heritability [5, 6, 7, 8, 9, 10]. Current investigations in Sri Lanka are continuing on the effects of geochemical and occupation exposure, such as agrochemicals; climate change-associated issues, such as chronic dehydration and frequent exhaustion; and the combined effects of fluoride- and magnesium-rich hardwater, etc. [11, 12, 13, 14].
Over the past four decades, CKDmfo has been reported in several tropical countries, including in Central America (e.g., Nicaragua and El-Salvador), Balkan countries, Costa Rica, Egypt, Bangladesh, China, and Uddanam district of Andhra Pradesh, India, etc. [8, 15, 16]. The lack of focus, efficacious, and consistent governmental policies, chronic malnutrition, insufficient access to modern healthcare and escalating costs, and the changing needs in the remotely located communities seem to contribute to the genesis of CKDmfo in Sri Lanka and elsewhere [17].
This study was undertaken to fill the existing gap in knowledge regarding the cost and effectiveness of different interventions for preventing the disease, prioritize disease-prevention programs based on cost-effectiveness, and to identify key interventions needed to eradicate CKDmfo from the country. Currently, there is no data or literature available on estimates of the cost of CKDmfo prevention or eradication. Thus, this study was designed to fill this knowledge gap.
1.2. CKDmfo affecting tropical countries
A specific cause for this disease has been identified only in a handful of countries. In Bangladesh, the disease is triggered by ingestion of groundwater-based arsenic that predominantly cause genitourinary tract malignancies [13]. In the Balkan region (Serbia, Bulgaria, Romania, Croatia, and Bosnia) [14] and in China, aristolochic acid (Aristolochia indica) is suspected as a cause for this renal tubular disease [14, 15]. No cause has been identified in other countries, including ones in Mesoamerica (Nicaragua, El Salvador, etc.), India, Costa Rica, Egypt, and Sri Lanka, [4, 18, 19, 20, 21, 22].
Most of the affected countries are located within 1,000 km of the equator (tropical countries) and are economically poor; all have predominantly agriculture-based economies and malnutrition is common [23]. CKDmfo, a silent disease with chronic interstitial tubulo-nephropathy that exterminates approximately 30,000 people annually in CKDmfo affected countries; most victims are male farmers living in remote communities [4, 18, 19, 20, 21, 22]. This study consists of population-based data collection to develop effective strategies to prevent CKDmfo. As individual study subjects were not identified, ethics approval was deemed not required.
1.3. CKDmfo in Sri Lanka
In Sri Lanka, the disease is exclusively affecting the people living in the dry zonal regions; over 80% of which are farming communities. This fatal disease is not restricted among agricultural workers. These regions have little access to safe drinking water, education, modern medical facilitates, and effective preventive healthcare [24]. In Sri Lanka, currently three provinces covering approximately 30,000 km2 are affected, with 3.0 million people at risk of developing the disease [4, 16, 24, 25].
Although there are differences in types of crops and agrochemicals used in affected countries, many similarities exist [5, 16], including year-around hot and dry weather, poor soil drainage and intermittent floods, high prevalence of poverty and malnutrition, inadequate access to healthcare and sanitation, lower education and socioeconomic levels, and little access to clean water [4]. Detailed geographic information regarding disease spread has been published [16, 23]. Although CKDmfo has been present in Sri Lanka for over two decades, few or no effective preventative actions have been introduced [16, 26]—another reason for embarking on this research program.
2. Hypothesis
2.1. Vulnerability to the disease
Exposure to nephrotoxins, at levels higher than the maximum allowable limit (MAL) can cause renal damage. When the human body is exposed to multiple toxins, substantial damage can occur, even at levels lower than the MAL. The mechanism—the final common path leading to renal damage—seems to occur through excessive renal tubular oxidative stress [4]. Currently, no evidence exists for a microbial involvement, genetic predisposition or susceptibility to CKDmfo, but combination factors such as widespread micronutrient malnutrition, antioxidant deficiencies, and harmful personal habits leading to sustained chronic dehydration are likely to increase the susceptibility [4, 6, 18].
There are a number of factors that have not been investigated, including the effects of chronic infections (e.g., hantavirus and leptospirosis), changes of dietetic constituents (predominantly carbohydrate-rich (fructose) diets with insufficient proteins, antioxidants, and micronutrients), use of illicitly brewed alcohol, chronic dehydration, excessive use of over-the counter nephrotoxic medications (e.g., NSAIDs), and a host of recently acquired unhealthy behaviours [4, 6, 14, 18]. Current data strongly support a multifactorial aetiology for this disease; thus, the terminology of CKDmfo.
2.2. Expected impact of this study
Based on the systematic analyses of data collected over two-decades and using the trends, we identified eight factors most important to the genesis and propagation of CKDmfo that should be eliminated to achieve disease eradication from the country. Consequently, author focussed on evaluating the costs, cost-savings, and outcomes from implementation of the said intervention programs, designed to eliminate the negative effects from these factors causing and propagating the disease (Table 1). Identification and the weight given to these eight factors are based on the assessment from community-based pilot studies.
Table 1.
Key interventions that are needed and their effectiveness in eradicating CKDmfo.
| Interventional method | Effect of reduction of CKDmfo (weightage in %) |
|---|---|
|
47 |
|
17 |
|
11 |
|
8 |
|
7 |
|
4 |
|
3 |
|
2 |
|
1 |
While the factors studied may not be exhaustive, systematically reducing their negative impacts on the communities and people, will prevent the younger generation from acquiring this deadly disease. Moreover, those who have already contracted the CKDmfo (i.e., currently approximately, 260,000 people in affected regions) to potential causative agents and conditions, the proposed interventions (especially potable water) would give the opportunity for those with early stages of kidney damaged to regain renal functions through self-repair mechanisms. Such can re-establish normal renal functions, and thus, prevent developing into renal failure.
Importantly, this study also addressed the estimated cost reductions following implementation of the proposed preventative measures that fills the gaps in knowledge. Such knowledge-based cost–benefit relationships then can be incorporated into an effective disease eradication plan, budgeting, and prioritizing large-scale interventions for disease prevention. Assessments were provided on the beneficial economic impacts following removal of each “causative group/factors,” contributing to the genesis of CKDmfo. The above will have a major health and socioeconomic benefits not only for the region but also for the country.
3. Methods
3.1. Data modelling methods
An analytical model was developed to use with a broader set of data pertinent to causation of the disease and its prevention. The collected information is from publicly available data from ministries and governmental departments in Sri Lanka, from the Internet, and data collected by the author's group over the past 20 years. Currently, no longitudinal study nor database exists for disease prevention or eradication, or the related to cost or benefits of CKDmfo prevention (or for that matter for any chronic disease) in Sri Lanka. Nevertheless, the model and the principles used here can be adapted to suit most of the chronic disease in Sri Lanka and in other countries.
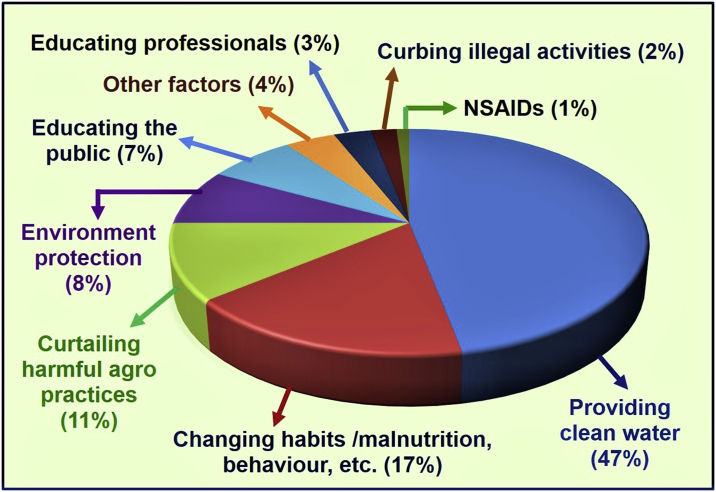
Gaps of longitudinal financial data were overcome by calculating and extrapolating data and in some cases, using the best estimates. Weighted estimates (please see Table 1) were generated for each variable based on its expected impact (generated through pilot studies and observations over several years) on estimated percentage impact on disease risk reduction. The estimated percentage impacts used in this study for each factor on disease incidence and risk reduction are illustrated in Fig. 1.
Fig. 1.
Effects of various interventions in reducing the incidence of CKDmfo. Impacts of various procedures and programs and their estimated contributions in reducing the incidence of CKDmfo in the country, are provided as percentages. Data are based on multiple realistic assumptions and approximate calculations.
3.2. Computations and projection of data to the future
To simplify the computation, the estimate provided ignored the “time value of money.” For example, if the currently low interest rates rise in the future, the cost of eradication efforts likely to be increase. To facilitate understanding, such was not levied on current calculations. To make computations easier, we assume that the incidence of CKDmfo and the dollars needed to spend to prevent the disease will remain proportionally constant over time. In reality, these rates and the value of the currency are likely to change. When it happens, estimated can be easily adjusted. In addition, the cost of implementation is higher at the beginning of the program and would gradually decrease as the incidence and the prevalence of the disease are lessened.
The cost for interventions per year was multiplied by prorated values for 15 to arrive at the total cost for the 15-year period, with the appropriate adjustments for the incremental reduction of funds and resources needed (estimated 30% reduction of costs in each three-year period). Costs are calculated in U.S. dollars for easier comparison of costs and the cost-effectiveness of interventions for the benefit of other countries affected by CKDmfo. Provision of actual values are beyond the scope of this manuscript.
3.3. Pitfalls of this study
This study has limitations, including the scarcity of real monetary values needed to derive estimated projected cost, a dearth of real-time data, not incorporation of the changing time value of money and the cost structure with the reduction of disease incidence, and the use of estimated weights. However, with accumulation of real-time data following prospectively implementing this program, estimates can be replaced with real values. That would allow easy adaptation and refinement of this dynamic, ongoing program.
Although the data analysis considered changing patterns in the spread of the disease and associated practical limitations, converting these factors to monetary values are only best estimates. With a specific disease caused by one aetiological agent, such as a parasitic, viral, or bacterial disease, elimination of the “causative factor” likely eradicates the disease. However, in a chronic disease such as CKDmfo that consists of multi-factorial aetiology, the relationships are not linear and impossible to come up with all interventions that are needed to eradicate it. However, in public health interventions in eradicating a disease, it only needs to overcome a significant proportion of causations and risk factors.
Other than the eight mentioned, all other factors were lumped into a separate category as ‘unknowns’ (Fig. 1). However, when more data emerge, this group can be subdivided for future calculations and preventative actions. Reasonable and customary administrative costs were included to cover the overhead and the programmatic costs. These cost estimates, savings, and effectiveness ratings are important for the model and for making it practical to use as an effective tool for prioritizing knowledge-based interventions based on cost-effectiveness. As mentioned, when new data emerges, broader program can be modify, cumulatively. This study does not contain personal identifications; thus, did not require an IRB approval or consent for publication. The author has no conflicts of interest and received no funds for this work.
4. Analysis
The analysis has taken into consideration (A) the cost of each intervention to reduce the incidence and the comparative effectiveness of the intervention—weightage factor (B) the expected cost savings from each intervention; (C) reduction of needed resources with time; and (C) potential synergistic interactions among interventions. The analysis attempted to encompass damage to natural and human−environmental capital, loss of growth, and opportunity costs.
4.1. The need for broader and interconnected interventions
Interventions and analyses for this study were taken in a combination sources of data, together with the predicted decline in the number of male farmers in CKDmfo-affected regions due to premature deaths [26] and the associated reduction of paddy and vegetable output in the CKDmfo-affected regions. No data are available on the outcomes from the current haphazard and inadequate disease-prevention practices undertaken by the successive governments [27, 28]. With time, the interventions proposed here are expected to reduce the disease management costs substantially; by an estimated 30%, in each three-year period.
4.2. The need for gatthering continuous data to optimize ongoing programs
Over the past two decades, we have conducted research programs, including surveys and market analyses [4, 6, 16, 26, 29]. Data from these studies were used in part to estimate the opportunity costs associated with loss of productivity and the potential cost savings. The disease management costs are from the department of health and cost-savings were calculated from implementing proposed interventions. It is noteworthy that no single intervention is adequate to eradicate CKDmfo from a country. Thus, it is essential to implement multiple parallel interventions to reduce the occurrence of CKDmfo and associated opportunity costs.
4.3. The importance of diligent follow-up
The major categories of cost that are expected to reduced through targeted interventions are (A) controlling the excess use of fertiliser and thus, the associated costs to farmers and government through education on appropriate usage and elimination of subsidies; (B) costs associated with managing and reducing public health risks; (C) cost of caring for patients with chronic diseases, such as CKDmfo (D) elimination of low productivity and opportunity costs; and (E) costs to the families caused by sickness and premature death of wage earners and societal and social disruptions.
The analyses taken into consideration that the reduction of the incidence of CKDmfo will occur through effective eight-fold interventions. This will reverse the current trend of declining productivity (e.g., reduction of rice production, caused by reduced cultivated land and manpower), as the region experiencing increasing shortage of middle-age male farmers due to disease burden and premature deaths, and migration to cities for job opportunities [26]. Preventing CKDmfo would allow villages to improve their living standards, productivity, socioeconomics and health status, societal harmony, stability and sustainability of the local economy, and improve the foreign exchange of the country [30].
5. Results
Evidence suggests the key sources of causative nephrotoxins are coming from drinking water [16, 31, 32]. Thus, it is not surprising that the provision of clean water is the most effective in reducing the incidence of CKDmfo. For example, a few villages that were prospectively provided with reverse osmosis (RO) purified potable water [33, 34] have led to a reduction of CKDmfo incidence by ∼45% over a 3-year period (reduced from 14% to 7.5%) among the adult population.
Moreover, those who are drinking from natural springs in the affected regions have the lowest prevalence of CKDmfo (i.e., less than 2% among the adults; author's unpublished data). Therefore, providing potable water to the entire affected and surrounding regions is a priority, as no other intervention, including healthcare is as effective as the provision of clean drinking water in reducing the disease incidence [4, 16].
5.1. Beneficial effects from drinking clean water
Drinking safe water not only prevents healthy people from contracting the disease but also reverses the disease in those with early stages of CKDmfo (i.e., below CKD, stage IIIa). Consequently, it will markedly reduce the healthcare burden and improves longevity and productivity [16]. Despite governmental claims, less than 20% of the population at risk has access to potable water and approximately, 50% have access to safe sanitary facilities.
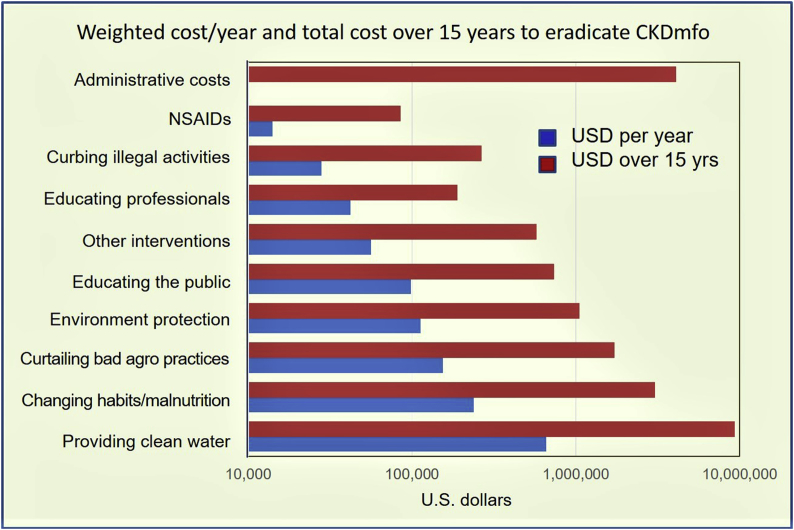
The estimated cost for the provision of clean water to the entire area is approximately $670,000/year. This is expected to reduce the CKDmfo incidence by approximately 45% (Fig. 2). Fig. 2 illustrates the costs for each intervention mentioned in Fig. 1, that is necessary to eradicate CKDmfo. Costs provided are per year for the 15-year duration that is needed to eradicate the disease. For ease in understanding the data, cost estimates needed to eradicate the disease are extrapolated and provided provided for one year intervals and for the entire 15 year duration.
Fig. 2.
Cost of eradicating CKDmfo using multiple parallel interventions. The costs associated with comparative effectiveness in reducing the incidence of CKDmfo with the proposed eight interventions are presented. Data are given as average cost per year and also total cost over the 15-year period to eradicate CKDmfo. Data are based on several assumptions and are approximate calculations (note: x-axis, is in semi-log scale). Costs are given in U.S. dollars.
This intervention, while costing $0.67 million/year, is expected to reduce healthcare and the associated costs by more than $15.6 million (1.8 billion Sri Lankan rupees) per year, even without including the opportunity cost. Thus, one-year's “savings” alone is adequate to implement clean water and other interventions to the entire 3.0 million, people at risk in the CKDmfo-affected regions. Thus, cost–benefit ratio from the provision of clean water alone is approximately 24-fold cost–benefit ratio. Drinking clean water prevents not only CKDmfo but also other waterborne diseases.
5.2. Effects of changing lifestyles and improving nutrition
Healthy lifestyle is not only a choice but also a privilege. Interventions through lifestyle changes, including nutrition is estimated to reduce the incidence of CKDmfo by 17%. Lifestyle changes needed in the said population include, consuming a nutritionally balanced diet, engaging in age-appropriate physical activities, and avoiding smoking, alcohol, and illicit drugs. In addition to CKD, these would also reduce the risks of other chronic diseases, such as type 2 diabetes, metabolic syndrome, obesity, and osteoporosis [35, 36].
Adopting a healthy diet that consists of a sustainable supply of locally produced fruits, vegetables, and lean animal products are highly desirable. However, these depend on the affordability and access to nutritious food. Our dietary studies indicated that in excess of 80% of calories consumed by villagers come from carbohydrates, with little protein, fat, and micronutrients. Considerable amounts of calories are originating form fructose that may have independent adverse effects on kidneys. The savings from sustainable lifestyle changes and improved nutrition on the reduction of CKDmfo are estimated to be $261,000/year (total savings of $3.6 million over a 15-year period).
5.3. Environmentally friendly agricultural practices and responsible use of agrochemicals
Currently, other than responsible for some suicides, there is no direct or credible scientific evidence that agrochemicals causing any known illness in Sri Lanka. Nevertheless, elimination of fertilizer subsidy (used to cost over 50 billion rupees/year for the government) and tightening the regulations related to marketing pesticide and their use will reduce their abuses and overuse. By doing so, both farmers and the government would save billions of rupees each year, otherwise wasted funds.
These actions would reduce environmental pollution [37], harm to fauna and flora, and the cost of food—all without reducing crop output, or negatively affecting the foreign exchange [5, 6]. As with deforestation and overfishing, the overuse of agrochemicals can be potentially harmful to the environment and all living beings. The estimated savings are approximately $3.0 million a year (11% cost savings) for the country (Fig. 2).
5.4. Protecting the environment (air, soil, and water)
Fertilizers, such as phosphate and nitrates have a limited capacity to retain fertilizer, such as nitrates and phosphates [38, 39, 40, 41]. Those not retained will run off with rainwater into ecosystem [42], leading to algae blooms and death of freshwater fish, miles away from the origin of contamination [39, 41]. Similarly, the regular practice of burning material, and emissions from coal-powered plants cause environmental harm. Resultant air pollution causes increase acid rains and incidence of allergies and certain chronic diseases, such as chronic pulmonary diseases and asthma.
Protecting natural resources and investing to protect the environment would have a long-term beneficial impact on socioeconomic conditions and reduce chronic diseases. In the longer term, controlling environmental pollution in the longer term will reduce a number of chronic diseases, including CKDmfo. This is estimated to reduce the governmental cost by approximately 8% per year, a savings of approximately $114,000/year (Fig. 2).
5.5. Educating the public, farmers, and vendors
In countries where there are governmental fertiliser subsidies (as it was used to be in Sri Lanka till recently), it is an incentive for farmers to overuse agrochemicals. Many farmers believe that greater use of agrochemicals improves crop output, but more is not always better [5]. Implementing responsible use of agrochemical, in connection with educating farmers and vendors in the safe use and adhering to the recommendation by the department of agriculture is in the longer-term expected to reduce CKDmfo incidence by 11%, leading to a savings of approximately $100,000/year.
5.6. Educating professionals and implementing holistic approaches
The Department of Agriculture (DoA), the Environmental Protection Authority and (EPA), the Central Environmental Authority (CEA) collectively have a responsibility, not only to regulate the uses of agrochemicals but also to educate farmers, professionals, and the public on responsible use and sustainable development. Efforts in environmental protection, controlling disease, and implementing the best practices for preventing CKDmfo are estimated to reduce CKDmfo by 8%, a savings of $42,000/year (Fig. 2).
All governmental officials including teachers, should be educated on environmental protection and disease control, and the best practices for preventing chronic diseases such as CKDmfo; and accordingly, should be empowered to impart knowledge to others [4, 23, 26, 30]. In this regard, the novel school program of teaching about the environment and nature protection and mindfulness in school curricula by the Sathipasala foundation (https://www.satipasala.org/) in Sri Lanka, in the longer-term, expected to have a major long-term benefit for a country.
5.7. Effects of curbing the use of illicit alcohol, tobacco, and drugs, and abuse of pain relievers
Frequent consumption of illicitly brewed (frequently contaminated) alcohol, smoking locally grown tobacco that contains higher amounts of heavy metals and fluoride, and taking over-the-counter pain killers all could contribute to the genesis of CKDmfo [4, 10, 43]. Because education alone is unlikely to curb such habits, healthcare professionals and other stakeholders should be involved to promote sustainable behavioural changes with appropriate incentives to change their lifestyles [44, 45]. Regulating the consumption of alcohol and tobacco and eliminating drug abuse are estimated to have approximately 2% effect on reducing CKDmfo, with a savings of $28,000/year.
6. Discussion
6.1. Costs of each intervention and cost-savings, for CKDmfo eradication
6.1.1. Estimated general costs associated with CKDmfo
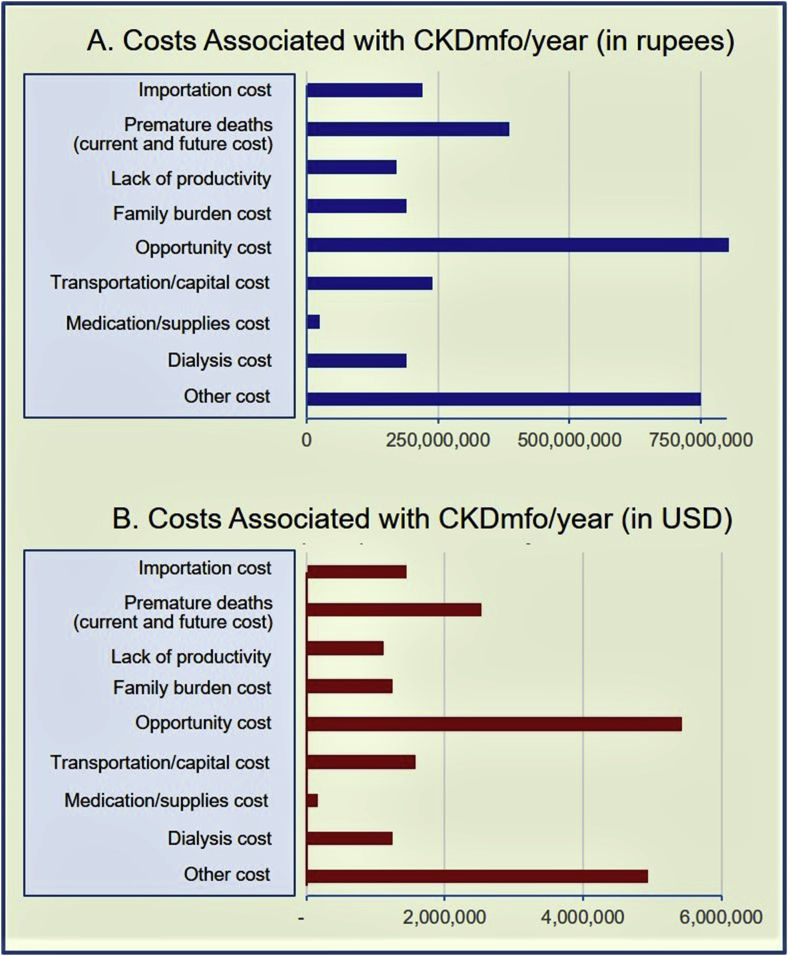
In addition to the medical care and patient management costs for the government, CKDmfo is associated with several other types of costs, including cost for the family and the society and the opportunity costs. Average annual costs as of end of 2018 are illustrated in Fig. 3, (A) in rupees and in (B) in United States dollars.
Fig. 3.
The current annual costs associated with CKDmfo in Sri Lanka: Average current costs are illustrated in (A) rupees and in (B) U.S. dollars, per-year basis.
6.1.2. Costs and cost savings following implementing proposed projects to eradicate CKDmfo
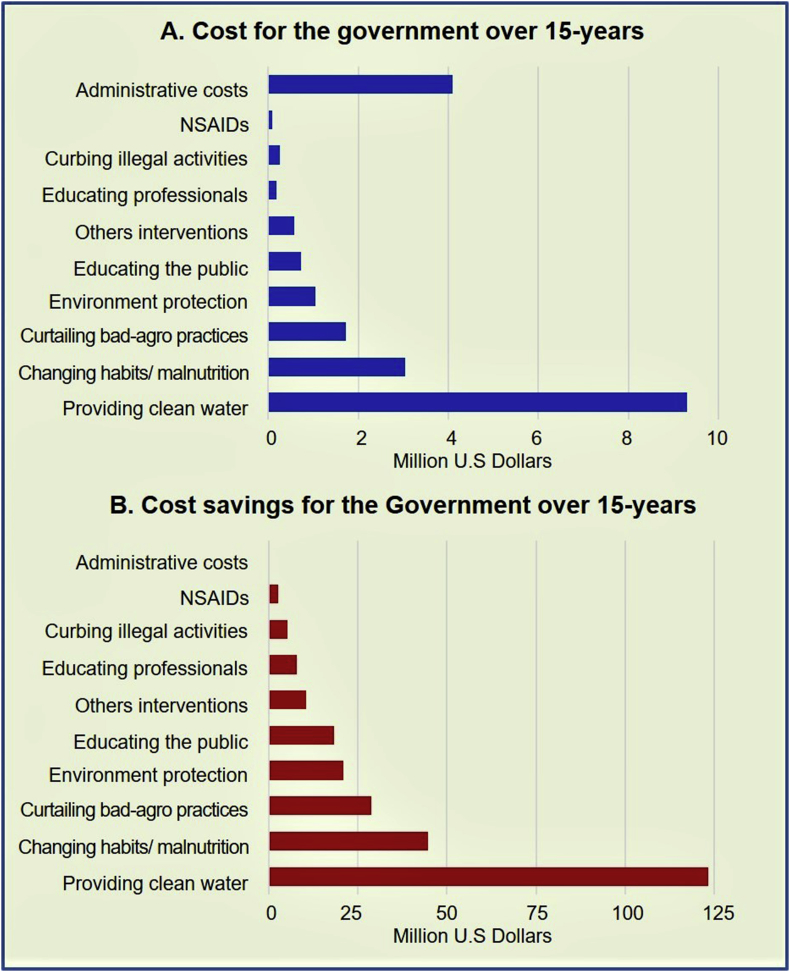
The costs related to implementing eight parallel projects and the resultant cost savings for the government are illustrated in Fig. 4. Fig. 4A demonstrates the societal cost the taxpayers burden (the government) for eradicating CKDmfo over a 15-year period, and Fig. 4B illustrates the total cost savings over the same period.
Fig. 4.
Costs of interventions and the costs savings for the government over a 15-year period: (A) Costs for the government for implementation of eight simultaneous programs and (B) cost savings for the government achievable through individual interventions over a 15-year period (x-axis, semi-log scale) are presented. The total estimates are given in U.S. dollars for the entire 15-year period necessary to eradicate CKDmfo.
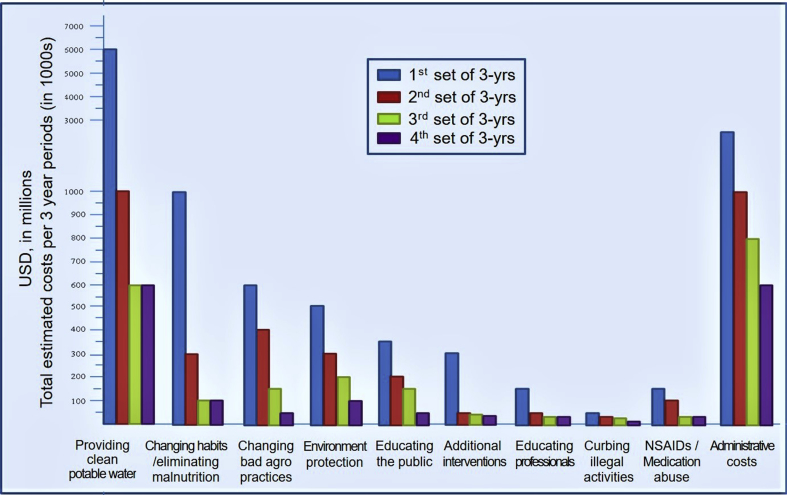
With effective interventions, it is expected that the disease incidence will reduce with time. We envisage that for each 3-year period, the costs of implementing the proposed programs will be reduced by 30%. A stepwise, noticeable reduction of the cost is anticipated. Fig. 5 illustrates the total estimated costs provided for each 3-year period, following executing the eight specific interventions. In the model, we have included the expected cost reduction with time, for each category, following intervention programs leading to reduced incidence and eventually, the prevalence of CKDmfo.
Fig. 5.
Estimated cost to eradicate CKDmfo from the country: Data presented in 3-year segments of costs in U.S. dollars for each of eight key intervention programs to prevent and eradicate CKDmfo. The expected reductions of costs with each 3-year time segment are also shown.
Fig. 5 visually portrays how the cost for a proposed intervention will reduce over time. These estimates are valuable for medium-term budgeting, planning, and implementing projects. Costs are categorized into groups of 3-year periods and were based on trend analysis data, extrapolated for 15 years, and the time estimated to be required to eradicate the disease. Weighted average costs over 3-year periods are illustrated in Fig. 5.
6.2. Practical steps that should be taken to prevent CKDmfo
At the current rate of premature death among the males who contracted CKDmfo, the number expected to live in the North Central Province (NCP) in 2,048 will be low, between 12% and 15% of that in 2,012 [26]. If no effective intervention is implemented to prevent the disease as happening currently, a marked change of demography is expected. This is unjustified, as the CKDmfo is preventable. Despite this impending personnel and socioeconomic tragedy, governmental interventions are being implemented too little, too slowly, hampered by the lack of political will.
6.2.1. Actions that are necessary for disease prevention
Data indicate the importance of parallel interventional approaches for preventing CKDmfo. Table 2 displays a multitude of simultaneous, real-time, preventative interventions needed to eradicate the disease. Because there are synergistic interactions among the proposed interventions, the maximum benefit is expected only when these measures are implemented concurrently.
Table 2.
Strategies, actions, and procedures that need to be carried out to prevent and eliminate CKDmfo in Sri Lanka.
| Item | Process | Action needed |
|---|---|---|
| 1 | Provision of clean, potable water |
|
| 2 | Changing agricultural practices |
|
| 3 | Changing lifestyles |
|
| 4 | Protection of the environment |
|
| 5 | Education of the public |
|
| 6 | Public health measures |
|
| 7 | Curative measures |
|
| 8 | Education of professionals |
|
| 9 | Curbing drug use, illegal alcohol and tobacco |
|
| 10 | Education of farmers and vendors on appropriate use of agrochemicals |
|
| 11 | Research program |
|
In addition to the standardisation of water sample collection and testing, and provision of clean water, facilities should be provided locally, for water and soil testing, free or at an affordable cost. In addition to installing interim RO or a similar efficacious water purification system, the National Water Supply Board must incorporate new technology to the existing local water treatment facilities to assure the removal of all potential nephrotoxins from drinking water.
However, the current, method routinely implemented by the national water supply and drainage board (i.e., size exclusion, alum, and chorine treatments) in Sri Lanka is insufficient and incapable of removeing the offending nephrotoxins from water. Thus, unless modern water purification methods are incorporated into new facilities in the CKDmfo affected regions, to generate drinking water (but this is not what the water board is currently planning and funded for). If this is the case, it will have little or no effect on improving the quality of drinking water to prevent this fatal chronic renal disease. All water purification methods to be used in the CKDmfo-affected regions must be capable of removing “all nephrotoxic contaminants,” to levels well below the MAL [34, 46].
6.2.2. Prioritization of plan of action
As discussed above, provision of potable water has the most impact of reducing the incidence of CKDmfo. However, to reduce CKDmfo incidence, water purification systems used must eliminate all nephrotoxins from drinking water, including agrochemicals, fertiliser, heavy metals, fluoride, hardness, salts, and so forth [4, 16]. The purification method must be rugged and cost-effective, and a turnkey operation that producing clean, potable water.
To be fair to these economically poor communities and encourage its usage, clean water should be provided, free of charge to community centres, schools, temples, and for those who cannot afford the cost of purified water. However, a reasonable charge (e.g., one rupee/litre or less: = $0.50 for 100 litre of RO water) should be made available to all other customers for drinking and cooking water, allowing to cover the labour and the maintenance costs. Currently the only method that guarantees to produce water devoid of nephrotoxins is the RO [34, 47].
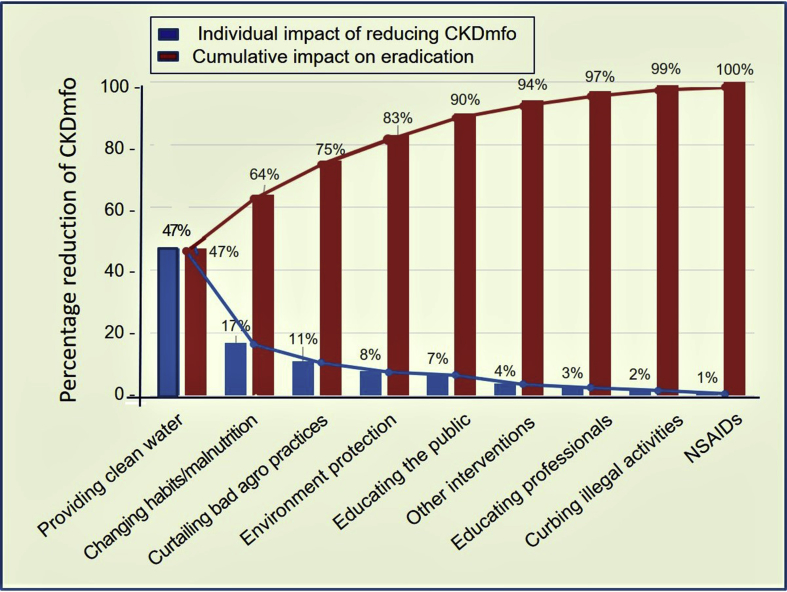
Data indicate that the effects of each proposed interventions are additive. Fig. 6 is a Pareto chart that demonstrates the cumulative effects of each interventions in preventing CKDmfo.
Fig. 6.
Pareto chart of the cumulative effects of each intervention on the prevention of CKDmfo. The first four key interventions collectively, are estimated to achieve 83% reduction in the disease. From a public health point of view, exceeding the reduction of incidence of CKDmfo by 80% can be considered sufficient to lead the way for eventual eradication of the disease. Blue bars = disease prevalence; red bars = cumulative impact on disease prevention.
6.3. Recommendation
The origin (the causation) of CKDmfo is multifactorial. Poverty-driven malnutrition, unhealthy lifestyles and habits, excessive use of agrochemicals, and ineffective governmental policies, all can be intricately interconnected [16, 35]. These factors likely to make individuals vulnerable, while chronic and prolonged ingestion of nephrotoxins contribute to precipitate CKDmfo. To prevent and eradicate a disease, it is important to understand the root cause(s) of the disease. Although the causative agent(s) are still unknown and likely to be multifactorial, the quality of drinking water (and not food) has been clearly identified as the major conduit for developing CKDmfo.
This study revealed the need for multiple population-level disease-eradication strategies. With 45% effectiveness, the single most-effective measure for preventing CKDmfo is the provision of clean water. Once implemented across the affection regions, it estimated to save the government more than $9.0 million/year of the costs associated with CKDmfo [5, 16]. However, clean water alone will not completely prevent the disease or be able to eradicate it. Thus, other measures described must be implemented simultaneously.
In Sri Lanka, the governmental costs for caring for patients with CKDmfo plus (lost)opportunity costs are approximately $19.7 million/year (equivalent to 3 billion Sri Lankan rupees/year); moreover, this is a recurring cost. Providing clean water to the entire affected region would be a one-time investment of $9.5 million that would reduce the overall disease incidence by 45%. Thus, investing less than 20% of one year's governmental expenses in addressing this disease, is sufficient to provide potable water to all affected regions in the country, and reducing the CKDmfo incidence by 45%.
As with other public health models, the described holistic approach necessitates simultaneously implementation of multiple programs across the region, to reduce the incidence and eventually eliminate the disease from the country [35]. This would markedly reduce the cost to affected families, communities, the public, and the government, and increase food security, boost the nation's economy and prosperity. Implementation of these measures would make building new renal hospitals and centralized renal dialysis facilities (costing the government hundreds of million dollars) redundant. The overall cost for preventing this deadly disease is fully offset by the cost recovery within the first 3 years (Fig. 2).
However, intervention programs must be flexible and modifiable as new real-time data accumulate. Ongoing analysis should lead to streamlining knowledge-based, proactive intervention strategies (rather than reactive approaches taken for disease management) to achieve the final goal of disease eradication. This goal is feasible, within 15-years of program implementation as illustrate in Fig. 6. Moreover, GIS-based effective, prospective surveillance program will facilitate, accurately studying the elements in water, air and soil systems, nephrotoxins and other toxins, and independent and unknown variables, over time in a wide geographical area. This approach will pave the path to identify the causes of the disease, allowing further refinements to the modes of prevention and eradication programs.
As the disease incidence declines, management and programmatic costs will progressively decrease. Disease prevention reduces treatment-associated costs, keeping people healthy, and thus, improving productivity and opportunity costs. Achieving these goals requires the understanding, cooperation, and collaboration of all stakeholders, including farmers and professionals, in domains such as healthcare, the environment, public and private entities, and policy makers. Implementing parallel interventions is essential to enhance their efficiency and synergy, thus maximising outcomes with least cost. The methodology described here, and the conclusions made, are applicable to most chronic disease, and particularly so in all tropical countries that are affected by CKDmfo.
7. Conclusions
The causes of CKDmfo are multifactorial, so multipronged and multi-disciplinary solutions are needed to eradicate the disease. It is unlikely that a single solution or one government department or a ministry could overcome the CKDmfo epidemic in a particular country. Agribusiness, government departments, including the DoA, EPA, and various ministries should work in cooperation to incentivise farmers to use agrochemicals responsibly, while popularizing hi-yielding hybrid methods requiring less water to increase agricultural output and minimise pollution.
Reverting to traditional or organic agriculture is not a solution. Such would not only create a serious food scarcity and food insecurity in the country but also will have no effect on reducing the incidence of CKDmfo. The inability to identify causes to-date, is in part due to the lack of focus and funding, non-hypotheses driven programs, and insufficient collaborations. These failures prolonging the agony of affected people and their families, people continue to develop this fatal disease, and the societal and governmental costs will escalate.
There is no scientific evidence that CKDmfo/CKDu is caused by the use of pesticides or agrochemicals. However, creation of food, clean water, and energy security for the region and the country is a must. This can be achieved within he next five years by strategically using modern technology and not going back in time. Food cultivated in Sri Lanka is safe for consumption, but harvest per hectare is insufficient to feed the population. Savings from fertilizer subsidies, reduction of purchasing petroleum products, etc., can be utilize for enhance agricultural productivity (the use of modern, hybrids and high-yielding varieties), protection of the environment, and develop peace and security.
If the described interventions are fully implemented, CKDmfo can be eliminated from Sri Lanka within 15 years. The data and outcomes described are important to designing budgetary allocations, prioritization of interventions, and impacting policy decisions. These concepts and data provide a foundation for the prevention and eradication of CKDmfo from Sri Lanka and elsewhere. No medical treatment can prevent the incidence or spread of this environmental, occupational, and life-style-associated disease; thus, preventative measures should be prioritized. To achieve this, all stakeholders must work in cooperation. Prevention is the cure.
Declarations
Author contribution statement
Sunil J. Wimalawansa: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the manuscript.
Funding statement
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The author gratefully acknowledges initial contributions of Janeesha Warusawithana and the helpful comments of Eugene Heyden and Professor Rosa Oppenheim, Dean's Professor of Business and professor of statistics, vice-chair of the department of supply-chain management, Rutgers School of Business, New Jersey, U.S.A.
References
- 1.Weaver V.M., Fadrowski J.J., Jaar B.G. Global dimensions of chronic kidney disease of unknown etiology (CKDu): a modern era environmental and/or occupational nephropathy? BMC Nephrol. 2015;16:145. doi: 10.1186/s12882-015-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharma-wardena C.D. 2014. List of References Pertinant to Sri Lanka, Including CKDmfo.http://dh-web.org/place.names/posts/index.html#ckdu [Google Scholar]
- 3.WHO-CKDu-Final Report. Jayathilaka N.M.P., Mendis S., Mehta F.R., Dissanayake L.J., Janakan N. World Health Organization; Sri Lanka: 2013. WHO Sri Lanka CKDu Report: Chronic Kidney Disease of Unknown Aetiology (CKDu): a New Threat to Health.http://dh-web.org/place.names/posts/index.html#ckduhttp://www.lankaweb.com/news/items12/WHO%20final%20report%20on%20CKDu%20SL.pdf Colombohttp://dh-web.org/place.names/posts/index.html#ckdu Available from: [Google Scholar]
- 4.Wimalawansa S.J. Escalating chronic kidney diseases in Sri Lanka: causes, solutions and recommendations. Environ. Health Prev. Med. 2014;19(6):375–394. doi: 10.1007/s12199-014-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wimalawansa S.A., Wimalawansa S.J. Impact of changing agricultural practices on human health: chronic kidney disease of multi-factorial origin in Sri Lanka. Wudpecker J. Agric. Res. 2014;3(5):110–124. [Google Scholar]
- 6.Wimalawansa S.A., Wimalawansa S.J. Agrochemical-related environmental pollution: effects on human health. Glob. J. Biol. Agric. Health Sci. 2014;3(3):72–83. [Google Scholar]
- 7.Edirisinghe E., Manthrithilake H., Pitawala H., Dharmagunawardhane H.A., Wijayawardane R.L. Geochemical and isotopic evidences from groundwater and surface water for understanding of natural contamination in chronic kidney disease of unknown etiology (CKDu) endemic zones in Sri Lanka. Isot. Environ. Health Stud. 2017:1–18. doi: 10.1080/10256016.2017.1377704. [DOI] [PubMed] [Google Scholar]
- 8.Rango T., Jeuland M., Manthrithilake H., McCornick P. Nephrotoxic contaminants in drinking water and urine, and chronic kidney disease in rural Sri Lanka. Sci. Total Environ. 2015;518–519:574–585. doi: 10.1016/j.scitotenv.2015.02.097. [DOI] [PubMed] [Google Scholar]
- 9.Senevirathna L., Abeysekera T., Nanayakkara S., Chandrajith R., Ratnatunga N., Harada K.H. Risk factors associated with disease progression and mortality in chronic kidney disease of uncertain etiology: a cohort study in Medawachchiya, Sri Lanka. Environ. Health Prev. Med. 2012;17(3):191–198. doi: 10.1007/s12199-011-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanayakkara S., Senevirathna S., Harada K.H., Chandrajith R., Hitomi T., Abeysekera T. Systematic evaluation of exposure to trace elements and minerals in patients with chronic kidney disease of uncertain etiology (CKDu) in Sri Lanka. J. Trace Elem. Med. Biol. 2019;54:206–213. doi: 10.1016/j.jtemb.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Redmon J.H., Elledge M.F., Womack D.S., Wickremashinghe R., Wanigasuriya K.P., Peiris-John R.J. Additional perspectives on chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka--lessons learned from the WHO CKDu population prevalence study. BMC Nephrol. 2014;15:125. doi: 10.1186/1471-2369-15-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wasana H.M., Aluthpatabendi D., Kularatne W.M., Wijekoon P., Weerasooriya R., Bandara J. Drinking water quality and chronic kidney disease of unknown etiology (CKDu): synergic effects of fluoride, cadmium and hardness of water. Environ. Geochem. Health. 2016;38(1):157–168. doi: 10.1007/s10653-015-9699-7. [DOI] [PubMed] [Google Scholar]
- 13.Wasana H.M., Perera G.D., De Gunawardena P.S., Bandara J. The impact of aluminum, fluoride, and aluminum-fluoride complexes in drinking water on chronic kidney disease. Environ. Sci. Pollut. Res. Int. 2015;22(14):11001–11009. doi: 10.1007/s11356-015-4324-y. [DOI] [PubMed] [Google Scholar]
- 14.Jayasekara K.B., Kulasooriya P.N., Wijayasiri K.N., Rajapakse E.D., Dulshika D.S., Bandara P. Relevance of heat stress and dehydration to chronic kidney disease (CKDu) in Sri Lanka. Prev. Med. Rep. 2019;15:100928. doi: 10.1016/j.pmedr.2019.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lunyera J., Mohottige D., Isenburg M.V., Jeuland M., Patel U.D., Stanifer J.W. CKD of uncertain etiology: a systematic review. Clin. J. Am. Soc. Nephrol. 2016;11(3):379–385. doi: 10.2215/CJN.07500715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wimalawansa S.J. The role of ions, heavy metals, fluoride, and agrochemicals: critical evaluation of potential aetiological factors of chronic kidney disease of multifactorial origin (CKDmfo/CKDu) and recommendations for its eradication. Environ. Geochem. Health. 2016;38:639–678. doi: 10.1007/s10653-015-9768-y. [DOI] [PubMed] [Google Scholar]
- 17.Wimalawansa SJ http://www.sundaytimes.lk/150503/sunday-times-2/kidney-disease-in-ncp-focus-on-prevention-147418.html: Sunday Times, Sri Lanka; 2015[.
- 18.Ramirez-Rubio O., Brooks D.R., Amador J.J., Kaufman J.S., Weiner D.E., Scammell M.K. Chronic kidney disease in Nicaragua: a qualitative analysis of semi-structured interviews with physicians and pharmacists. BMC Public Health. 2013;13:350. doi: 10.1186/1471-2458-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefanovic V., Radenkovic S., Cukuranovic R., Kostic S. Balkan endemic nephropathy. Slowed progression of kidney disease by avoidance of etiological factors. Nephron. 1999;83(1):85–86. doi: 10.1159/000045477. [DOI] [PubMed] [Google Scholar]
- 20.Rajapurkar M.M., John G.T., Kirpalani A.L., Abraham G., Agarwal S.K., Almeida A.F. What do we know about chronic kidney disease in India: first report of the Indian CKD registry. BMC Nephrol. 2012;13:10. doi: 10.1186/1471-2369-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J., Zhang L., Wang W., Wang H. China national survey of chronic kidney disease working G. Association between aristolochic acid and CKD: a cross-sectional survey in China. Am. J. Kidney Dis. 2013;61(6):918–922. doi: 10.1053/j.ajkd.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Voice T.C., McElmurry S.P., Long D.T., Dimitrov P., Ganev V.S., Peptropoulos E.A. Evaluation of the hypothesis that Balkan endemic nephropathy is caused by drinking water exposure to contaminants leaching from Pliocene coal deposits. J. Expo. Sci. Environ. Epidemiol. 2006;16(6):515–524. doi: 10.1038/sj.jes.7500489. [DOI] [PubMed] [Google Scholar]
- 23.Wimalawansa S.J. Agrochemicals and chronic kidney disease of multifactorial origin: environmentally induced occupational exposure disease. Int. J. Nephrol. Kidney Fail. 2015;1(2):1–9. [Google Scholar]
- 24.Wimalawansa S.J. Escalating chronic kidney diseases of multi-factorial origin (CKD-mfo) in Sri Lanka: causes, solutions, and recommendations-update and responses. Environ. Health Prev. Med. 2015;20(2):152–157. doi: 10.1007/s12199-015-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wanigasuriya K. Update on uncertain etiology of chronic kidney disease in Sri Lanka's north-central dry zone. MEDICC Rev. 2014;16(2):61–65. doi: 10.37757/MR2014.V16.N2.10. [DOI] [PubMed] [Google Scholar]
- 26.Wimalawansa S.J., Wimalawansa S.A. Chronic kidney disease of multifactorial origin (CKDmfo) in Sri Lanka: escalating incidence and long-term survival estimates. J. Nephrol. Urol. Res. 2015;22(4):1–17. [Google Scholar]
- 27.Wimalawansa S.A., Wimalawansa S.J. Environmentally induced, occupational diseases with emphasis on chronic kidney disease of multifactorial origin affecting tropical countries. Ann. Occup. Environ. Med. 2016;28:33. doi: 10.1186/s40557-016-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wimalawansa S.J. Organization of Professional Association; Colombo Sri Lanka: 2017. Epidemic of Chronic Kidney Disease of Unknown Origin in Sri Lanka: what Really Causing it? Guidance for Eradication. World Water Day Symposium 2017. [Google Scholar]
- 29.Weeraratne S., Wimalawansa S.J. A Major irrigation project (Accelerated Mahaweli Programme) and the chronic kidney disease of multifactorial origin in Sri Lanka. Int. J. Environ. Agric. Res. 2015;1(6):16–27. [Google Scholar]
- 30.Aquastat. Country Profile: Sri Lanka. Food and Agriculture Organization of the United States. Report 37. 2012. http://www.fao.org/nr/water/aquastat/countries_regions/lka/index.stm Available from: [Google Scholar]
- 31.Chandrajith R., Nanayakkara S., Itai K., Aturaliya T.N., Dissanayake C.B., Abeysekera T. Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: geographic distribution and environmental implications. Environ. Geochem. Health. 2011;33(3):267–278. doi: 10.1007/s10653-010-9339-1. [DOI] [PubMed] [Google Scholar]
- 32.Nobel A.A.P., Manthrithilake H., Arasalingam S. International Water Management Institute (IWMI); 2014. Review of Literature on Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Colombo, Sri Lanka. Contract No.: IWMI working paper 158. [Google Scholar]
- 33.Glater J. The early history of reverse osmosis membrane development. Desalination. 1998;117:297–309. [Google Scholar]
- 34.Wimalawansa S.J. Purification of contaminated water with reverse osmosis: effective solution of providing clean water for human needs in developing countries. J. Emerg. Technol. Adv. Eng. 2013;3(12):75–89. [Google Scholar]
- 35.Wimalawansm S.J. Strategic framework for managing non communicable diseases: preventing chronic kidney disease of multifactorial origin (CKDmfo/CKDu) as an example. Chronic Dis. Int. 2015;2(2):1018. [Google Scholar]
- 36.Wimalawansa S.J. Stigma of obesity: a major barrier to overcome. J. Clin. Transl. Endocrinol. 2014;1:73–76. doi: 10.1016/j.jcte.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wimalawansa S.J. Escalating chronic kidney diseases of multi-factorial origin in Sri Lanka: causes, solutions, and recommendations. Environ. Health Prev. Med. 2014;19(6):375–394. doi: 10.1007/s12199-014-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sri Lanka Nature Forum. Nugegoda, Sri Lanka2008. Caring for Water; p. 161.
- 39.Wimalawansa S., Wimalawansa S.J. Protection of watersheds, and control and responsible use of fertiliser to prevent phosphate eutrophication ofreservoirs. Int. J. Res. Environ. Sci. 2015;1(2):1–18. [Google Scholar]
- 40.Wimalawansa S.J. Escalating chronic kidney diseases in Sri Lanka: causes, solutions and recommendations—update and responses. Environ. Health Prev. Med. 2015;20:152–157. doi: 10.1007/s12199-015-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dharmawardana M.W., Amarasiri S.L., Dharmawardene N., Panabokke C.R. Chronic kidney disease of unknown aetiology and ground-water ionicity: study based on Sri Lanka. Environ. Geochem. Health. 2014;37(2):221–231. doi: 10.1007/s10653-014-9641-4. [DOI] [PubMed] [Google Scholar]
- 42.Wimalawansa S.A., Wimalawansa S.J. Clean water, healthy environment, and preservation of watersheds: correct, enforceable policies are essential. Jacobs J. Hydrol. 2015;1(1):3–15. [Google Scholar]
- 43.Jayatilake N., Mendis S., Maheepala P., Mehta F.R. Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 2013;14(1):180. doi: 10.1186/1471-2369-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotir J.H., Smith C., Brown G., Marshall N., Johnstone R. A system dynamics simulation model for sustainable water resources management and agricultural development in the Volta River Basin, Ghana. Sci. Total Environ. 2016;573:444–457. doi: 10.1016/j.scitotenv.2016.08.081. [DOI] [PubMed] [Google Scholar]
- 45.Walley P., Silvester K., Mountford S. Health-care process improvement decisions: a systems perspective. Int. J. Health Care Qual. Assur. Inc. Leadersh Health Serv. 2006;19(1):93–104. doi: 10.1108/09526860610642618. [DOI] [PubMed] [Google Scholar]
- 46.Greenlee L.F., Lawler D.F., Freeman B.D., Marrot B., Moulin P. Reverse osmosis desalination: water sources, technology, and today's challenges. Water Res. 2009;43(9):2317–2348. doi: 10.1016/j.watres.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Wimalawansa S.J. Reverse osmosis: a cost-effective, interim solution to overcome water-pollution related human diseases. Eur. J. Biomed. Pharm. Sci.. 2018;5(2):988–998. [Google Scholar]