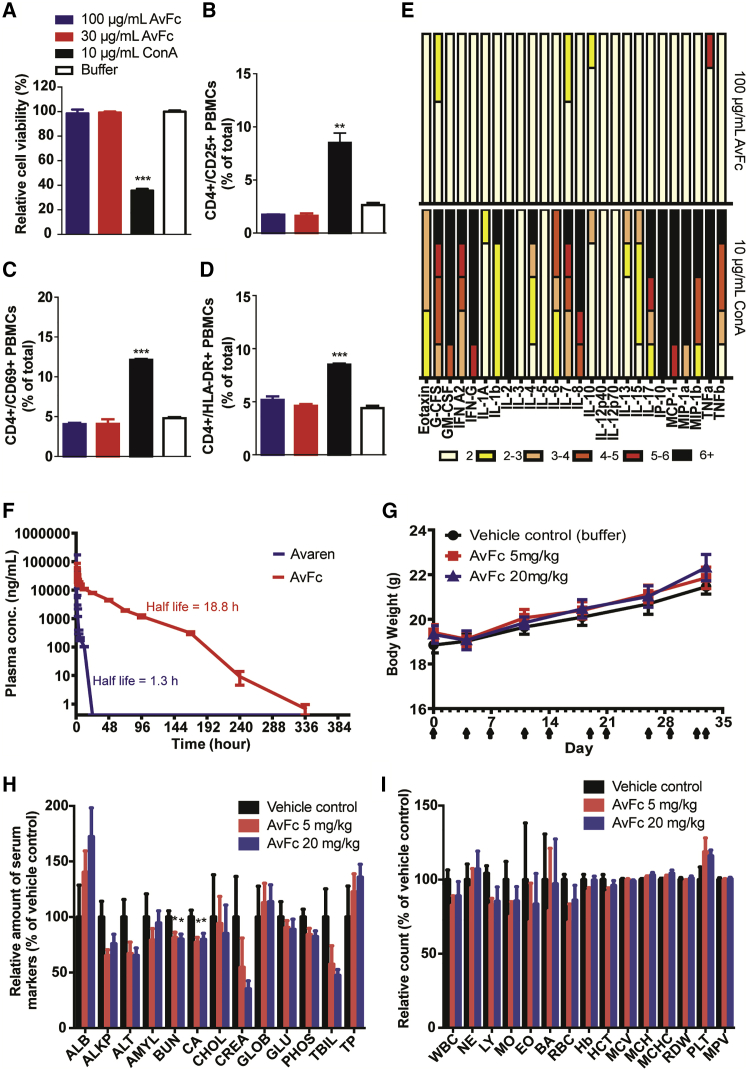
Figure 5.
AvFc Lacks Major Toxicity in In Vitro and Animal Models
(A) Human PBMC viability assessed by flow cytometry after staining with propidium iodide (PI). (B–D) Analysis of PBMC activation. PBMCs were treated with a vehicle control, ConA, or AvFc and analyzed for CD25 (B), CD69 (C), and HLA-DR (D) after dual fluorescent staining. (E) Cytokine and chemokine secretion by PBMCs (from five different donors) stimulated with AvFc or ConA for 72 h, assessed by a multiplex bead array. Changes in expression levels are subdivided into 1- to 2-fold (white bars), 2- to 3-fold (yellow bars), 3- to 4-fold (apricot bars), 4- to 5-fold (red-orange bars), 5- to 6-fold (red bars), and >6-fold (black bars) increase. (F) Pharmacokinetic evaluation of Avaren and AvFc. Serum Avaren and AvFc concentrations were measured by specific immunoassays (see Materials and Methods) at different time points after single bolus dose administration via the tail vein. Data represent mean ± SEM obtained for each group (n = 4), and half-life values were derived using nonlinear regression (curve fitting) in the GraphPad Prism software. (G–I) Effects of AvFc repeated systemic dosing on body weight (G), blood chemistry (H), and complete blood count (I) in mice (n = 10). Blood chemistry and complete blood count data were analyzed 1 day after the last dose in the mouse repeated-dosing study. Values are relative to the vehicle control group (mean ± SEM). Actual measured values are shown in Tables S2 and S3. AvFc showed significantly lower BUN and Ca levels than the vehicle control (*p < 0.05; one-way ANOVA with Bonferroni’s posttests); however, these values remained within the normal range for mice (10–33 mg/dL for BUN and 8.0–15.5 mg/dL for Ca, according to the University of Louisville Pathology Laboratory).