Abstract
Primary hyperparathyroidism (PHPT) is a rare endocrine disease in the pediatric population. Sporadic parathyroid adenomas remain the most common cause of pediatric PHPT. Parathyroid carcinoma (PC) is an extremely rare cause of pediatric PHPT. We report a 16-year-old boy presenting with a nonhealing fragility fracture of the right leg along with florid features of rickets. Examination revealed a neck mass, mimicking a goiter. Biochemical findings were consistent with PHPT. Imaging was suggestive of a right inferior parathyroid mass infiltrating the right lobe of thyroid. The patient underwent en bloc surgical excision of the parathyroid mass along with the right lobe of thyroid. Histopathology was suggestive of a PC. He achieved biochemical remission with normalization of serum calcium and parathyroid hormone levels. At follow-up, there was no biochemical or imaging evidence of recurrence or metastasis. Genetic analysis revealed heterozygous germline deletion of CDC73. An extensive literature search on PC was conducted, with an emphasis on the pediatric population. Thirteen cases of pediatric PC were identified. The median age of presentation was 13 years; there was no sex predilection. All cases were symptomatic; 31% had a visible neck mass. The median serum calcium and intact parathyroid hormone levels were 14.3 mg/dL and 2000 pg/mL, respectively. All patients underwent surgical excision, with 27% showing metastatic relapse. Our findings indicate that the preoperative features that could point toward a diagnosis of PC in a child with PHPT are a tumor size of >3 cm, thyroid infiltration on imaging, and severe hypercalcemia at presentation.
Keywords: pediatric parathyroid carcinoma, rickets, primary hyperparathyroidism, hyperparathyroidism-jaw tumor syndrome
Primary hyperparathyroidism (PHPT) is an uncommon endocrine disease in the pediatric population. The prevalence of pediatric PHPT is two to three cases per 100,000 [1]. Sporadic parathyroid adenomas remain the most common cause of PHPT (up to 80%) in this age group. Other causes include various syndromes, such as multiple endocrine neoplasia (MEN) 1, MEN 2A, hyperparathyroidism-jaw tumor (HPT-JT) syndrome and familial isolated hyperparathyroidism, which includes cases with GCM2 germline mutation. Parathyroid carcinoma (PC) as a cause of pediatric PHPT is extremely rare, and only a handful of published case reports are available in the literature [2–13]. The two largest case series of pediatric PHPT to date (with 55 and 52 patients, respectively) did not report a single case of pediatric parathyroid carcinoma [14, 15]. In two systematic reviews on PHPT, parathyroid carcinoma contributes <1% of the cases, almost all being adult cases [16, 17]. In this context, we report parathyroid carcinoma in a boy who presented with a nonhealing fragility fracture, visible neck swelling, and rachitic features. On reviewing the literature, in retrospect, we were able to pin-point some clinical, biochemical, and radiological features that could have predicted the presence of PC in the index case.
1. Case Presentation
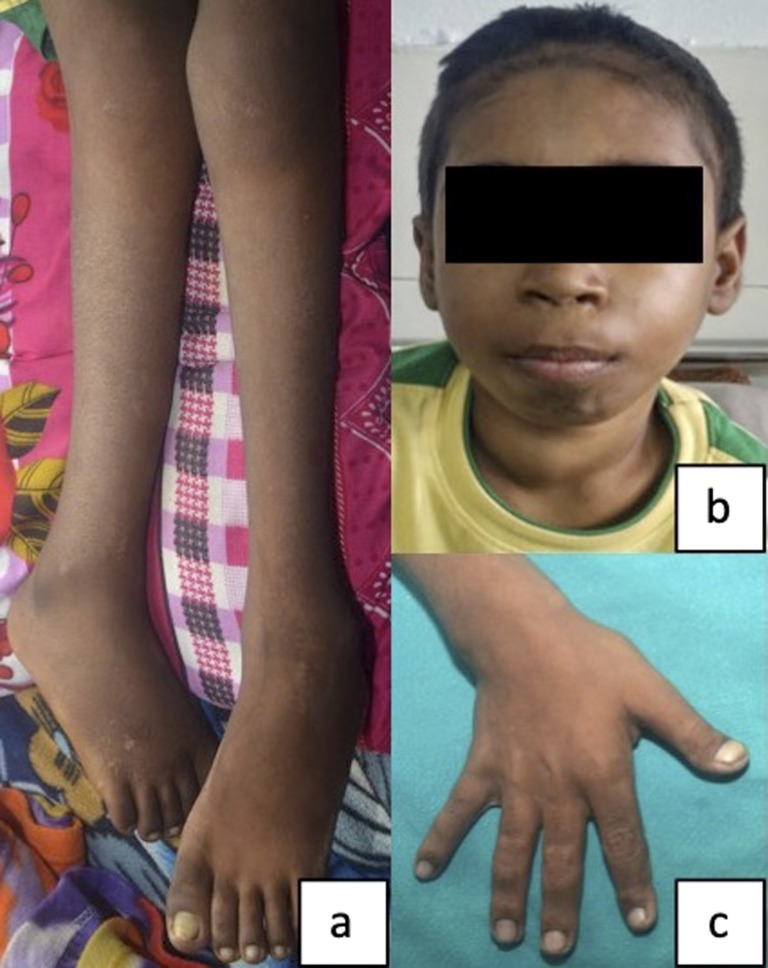
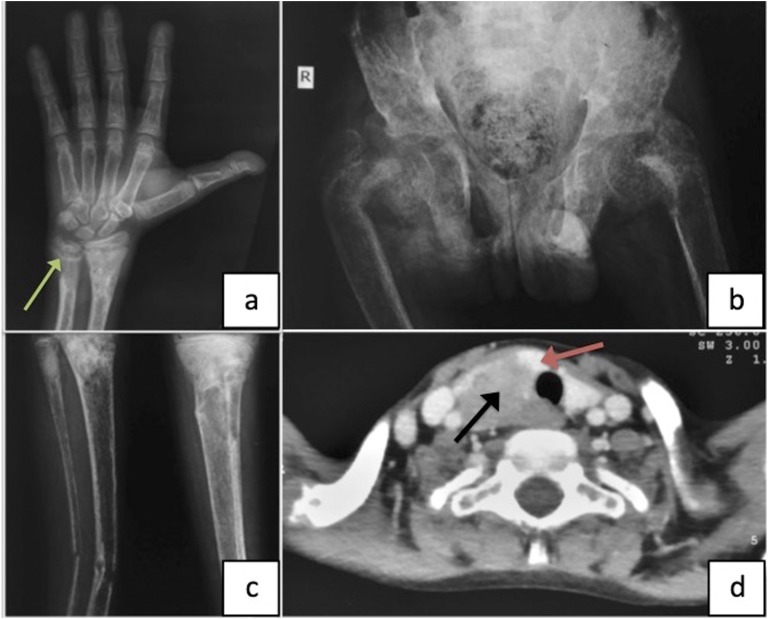
A 16-year-old boy presented to our institution with shortening and deformity (inwardly rotated) involving the right leg. Background history revealed that he had sustained a fragility fracture of right leg (tibia and fibula) 2 months back while climbing down from a vehicle. The injury was managed with a cast for 6 weeks. However, the fracture did not heal and led to the present deformity. On physical examination, his right leg was shortened and internally rotated (Fig. 1a). Tenderness and crepitus were present at the site of fracture. The patient also had a 3 × 4 cm, predominantly right-sided neck swelling. The swelling was firm and nontender and moved with deglutition (Fig. 1b). The patient was short for his age (height SDS −6.3) and was prepubertal (Tanner Stage A-P1G1). Other important clinical findings included an immature face, acro-osteolysis, kyphosis, pectus carinatum, and florid rachitic features (rachitic rosary; Harrison sulcus; and broad, tender ends of long bones) (Fig. 1c). Clinically, there was no evidence of jaw tumor (as seen in HPT-JT syndrome). A history of similar complaints in other family members was lacking. The patient’s initial biochemistry was as follows: serum-corrected calcium, 15.61 mg/dL (range, 8.8 to 10.2 mg/dL); serum phosphate, 2.88 mg/dL (range, 3.5 to 4.9 mg/dL); alkaline phosphatase, 2780 IU/L (range, 52 to 171 IU/L); intact parathyroid hormone (iPTH), 2028 pg/mL (range, 15 to 65 pg/mL); 25(OH) vitamin D, 5.93 ng/mL (target range, >20 ng/mL); serum creatinine, 0.7 mg/dL (range, 0.4 to 1.0 mg/dL); and normal thyroid function. Radiograph showed classical findings of PHPT (diffuse radiolucency, cortical thinning, intracortical tunneling, lytic lesions suggestive of brown tumors, salt and pepper appearance of skull). In addition, there was subperiosteal resorption of the phalanges, cupping and fraying of metaphysis, widening of the growth plate (Fig. 2a), bilateral coxa vara (Fig. 2b), and fracture of right tibia and fibula (Fig. 2c). Ultrasound of the neck revealed a multilobular, hypervascular, hypo-echoic mass posterior to and infiltrating into the right lobe of the thyroid. Ultrasound of the abdomen revealed bilateral renal calculi, staghorn calculus in right renal pelvis, and pancreatic calcifications. CT scan showed a 3.6 × 2.5 × 2.7 cm homogenously hypodense mass infiltrating postero-inferiorly into the right lobe of the thyroid and entering the tracheo-esophageal groove (Fig. 2d). 99mTc-methoxyisobutylisonitrile/single-photon emission CT scintigraphy confirmed this lesion to be a parathyroid mass. No jaw tumor was seen on CT in the mandible or maxilla.
Figure 1.
Clinical photograph of the patient showing (a) shortened and internally rotated right leg, (b) prominent right-sided neck swelling, and (c) broadened right wrist suggestive of rickets.
Figure 2.
Radiograph of the patient showing (a) subperiosteal resorption of the phalanges, widening of the growth plates of the right wrist, cupping and fraying of the distal ends of right radius and ulna (green arrow); (b) bilateral coxa vara; and (c) thinning of the cortices of leg bones, fracture of the right tibia and ulna, and well-defined lytic lesion involving the proximal part of left tibia. (d) CT of the neck (axial image) showing a 3.6 × 2.5 × 2.7 cm homogenously hypodense mass (black arrow) infiltrating postero-inferiorly into the right lobe of the thyroid (orange arrow).
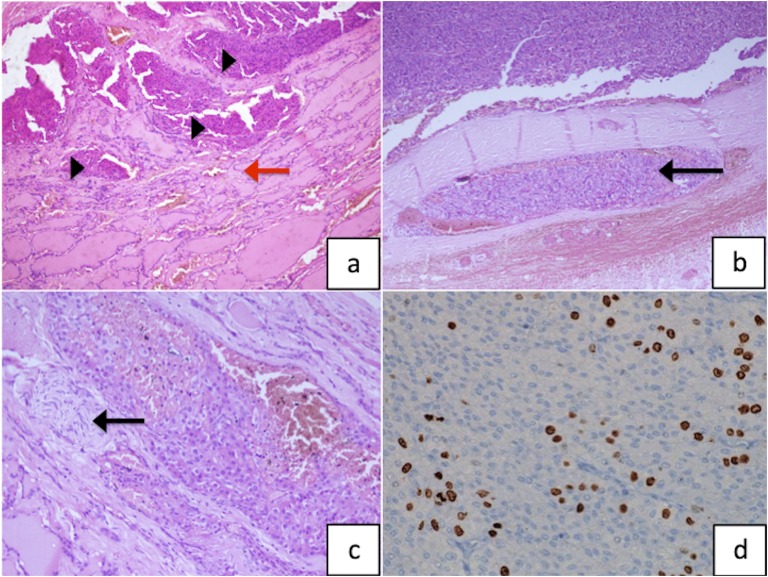
Hypercalcemia was managed with saline diuresis and subcutaneous calcitonin. Subsequently, the patient underwent open surgery under general anesthesia. The right inferior parathyroid mass was identified, which was seen infiltrating the right lobe of the thyroid. The mass along with the right lobe of the thyroid was removed en bloc. The rest of the three parathyroid glands were explored and were intraoperatively identified as being normal and were left in situ. No enlarged lymph node or tracheal, esophageal, or recurrent laryngeal nerve infiltration were seen. Postoperatively the patient developed hungry bone syndrome on day 2 and was managed with oral and intravenous calcium, oral calcitriol, and cholecalciferol supplementations. Postoperatively, his iPTH and calcium were reduced to 16.36 pg/mL and 8.9 mg/dL, respectively. Histopathology showed a nodular tumor tissue measuring 3 × 3 × 2.5 cm and weighing 10 g. The tumor tissue showed nests of moderately pleomorphic tumor cells with intervening fibrous septae. There was microscopic evidence of thyroid, vascular, and neural invasion. The Ki-67 index was documented to be 15% (Fig. 3a–d). The patient is being regularly followed at 3-month intervals, and at 1.5 years of follow-up, he is biochemically disease free. 18F-fluoro-2-deoxy-2-d-glucose positron emission tomography/CT performed at 3, 6, and 18 months of follow-up did not show FDG-avid loco-regional or metastatic disease. Radiologically, his rachitic features have markedly improved.
Figure 3.
(a) Photomicrograph showing parathyroid tumor tissue (black arrowheads) infiltrating the thyroid parenchyma causing follicular destruction (red arrow; hematoxylin and eosin; magnification ×100). (b) The tumor is seen causing vascular invasion (black arrow; hematoxylin and eosin; magnification ×100) and (c) perineural invasion (black arrow; hematoxylin and eosin; magnification ×200). (d) Ki-67 labeling index of the tumor tissue is 15% (magnification ×400).
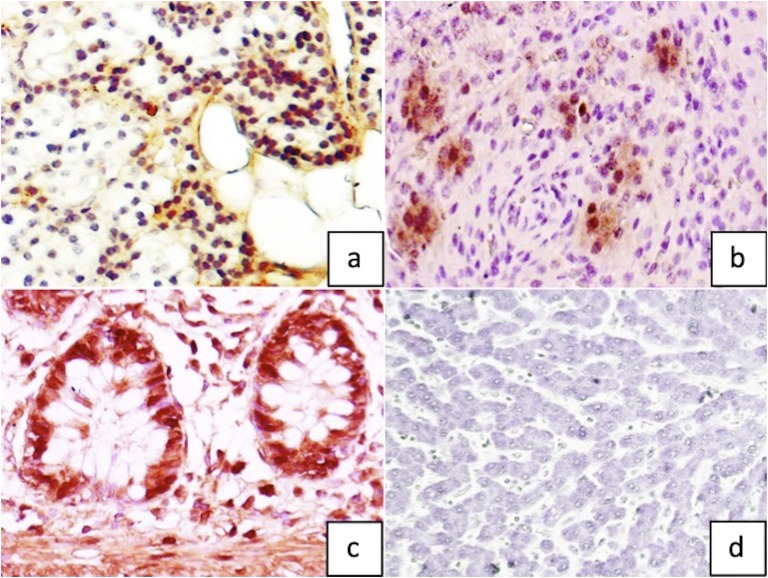
Genetic analysis for MEN 1 was negative. On Sanger sequence analysis of HRPT2 (CDC73), no pathogenic variant was detected. However, multiplex ligand-dependent probe amplification (MLPA®, SALSA MLPA P466 CDC73 probemix; MRC-Holland, Amsterdam, Netherlands) analysis revealed a heterozygous germline deletion of CDC73, with the deletion extending into the flanking regions (performed at Oxford Medical Genetics Laboratories, Oxford, United Kingdom). Immunohistochemistry for parafibromin (BSB-50, Bio SB, 1:200, Santa Barbara, CA) was performed on the tumor tissue, adjacent normal parathyroid tissue, positive (colonic mucosa) and negative (hepatocytes) controls. The tumor tissue showed sparse nuclear positivity for parafibromin, whereas the normal parathyroid tissue showed diffuse positivity (Fig. 4a–d). We will regularly follow the patient and will be vigilant on the emergence of clinical features suggestive of HPT-JT syndrome.
Figure 4.
Parafibromin immunohistochemistry (magnification ×400) showing (a) diffuse positivity in normal parathyroid tissue as compared with (b) sparse nuclear positivity in index parathyroid carcinoma. Representative images of (c) positive control (colonic mucosa) and (d) negative control (hepatocytes).
2. Review of the Literature
We conducted an extensive MEDLINE, PubMed, and Embase search using the terms “parathyroid cancer,” “parathyroid carcinoma,” “parathyroid malignancy,” “pediatric parathyroid carcinoma,” “pediatric parathyroid cancer,” “pediatric parathyroid malignancy,” “parathyroid carcinoma in a child,” “parathyroid cancer in a child,” and “parathyroid malignancy in a child.” In total, we could find only 13 cases (n = 7 female, n = 6 male) in the English language, highlighting the paucity of this diagnosis in the pediatric population. Our index case adds value to this small number of existing cases (Table 1). Among these cases, the median age at presentation was 13 years (range, 8 to 16 years). A family history of PHPT was present in three cases, and a proved genetic etiology of mutation in CDC73 was present only in case 13. Presenting complaints were reported in 13 cases, and all these cases were symptomatic in diverse ways. A neck mass was visible in four cases (31%). The median total serum calcium at diagnosis (reported in 12 cases) was 14.3 mg/dL (range, 12 to 20.7 mg/dL). Although PTH was reported in 12 cases, the corresponding value in case 4 was in Eq/mL (radioimmunoassay) and is not considered in this analysis. The median iPTH in the remaining 11 cases was 2000 pg/mL (range, 190 to 8368 pg/mL). In seven of the cases, the carcinoma was localized to inferior glands, and only one case was ectopic. Surgical records of only 12 patients are available; among these, six patients underwent simple excision, of whom one case had a follow-up hemithyroidectomy. Out of the six cases that underwent en bloc resection, five received it up front, and one case received it after simple excision. Follow-up has been reported in only 11 cases, of whom three cases (27%) had documented biochemical and image proven metastatic relapse. Our case had several unique findings, including parathyroid mass disguised as a goiter, severe hypercalcemia (>15 mg/dL), severe growth stunting, clinical features of rickets, pancreatic calcifications, and staghorn renal pelvis calculus. In retrospect, features such as size >3 cm, the presence of thyroid infiltration on ultrasonography, and severe hypercalcemia could have suggested a high preoperative likelihood of parathyroid cancer in this patient. Postoperatively, the presence of thyroid, neural, and vascular invasion and a high Ki-67 index proved the case as parathyroid carcinoma. Immunohistochemistry for parafibromin (the protein encoded by HRPT2) showed sparse nuclear positivity; genetic analysis revealed a heterozygous germline deletion of CDC73 extending to the flanking regions. Such deletions have been shown to be pathogenic in the development of parathyroid carcinomas [18].
Table 1.
Table Enlisting all 14 Pediatric Parathyroid Cancer Cases According to Year of Publication
| Case No. [Ref] | Age (y)/Sex | FH | Calcium/iPTH | Presenting Complaint | Location, Size (cm), Weight (g) | Surgery Performed | Relapse, Location | Follow-up | Tumor IHC/Genetic Analysis |
|---|---|---|---|---|---|---|---|---|---|
| 1 [2] | 13/NA | NA | NA | NA | NA, NA, NA | NA | NA | NA | NA/NA |
| 2 [3] | 13/F | NA | 15.9/NA | Bone pain, neck mass | RSPA, NA, 15 g | Simple excision | Yes, lung | Hypercalcemia | NA/NA |
| 3 [3] | 16/F | NA | 15.6/2000 | Anorexia, vomiting, renal calculi | LSPA, NA, 2 | Simple excision | No | Normocalcemia | NA/NA |
| 4 [4] | 15/M | NA | 16.0/800a | Pancreatitis, seizures | LIPA, 2×2, NA | Simple excision, rim of thyroid excised | No | NA | NA/NA |
| 5 [5] | 14/M | Yes | 13.2/3328 | Bone disease, myopathy, polyuria, polydipsia | RIPA, 3×3.5, NA | En bloc resection | No | Normocalcemia | NA/NA |
| 6 [6] | 15/M | NA | 20.7/358 | Neck mass, vomiting, fatigue, weight loss | LIPA, 3×2×2, 11.9 | En bloc resection | No | Normocalcemia | NA/NA |
| 7 [7] | 8/F | Yes | 14.3/190 | Renal calculi, lethargy, anorexia, neck mass | LIPA, 1.5×1×1, 2 | Simple excision | No | Normocalcemia | NA/MEN 1 analysis negative |
| 8 [8] | 10.5/M | Yes | 15.5/300 | Bone pain, fatigue, anorexia | Mediastinum, NA, NA | Mediastinal mass excision, thymectomy | No | Normocalcemia | NA/NA |
| 9 [9] | 14/F | No | 14.3/2792 | Polyarthralgia, myopathy, deformities, depression | RIPA, 2.5, NA | Right hemithyroidectomy | No | Normocalcemia | NA/NA |
| 10 [10] | 13/F | No | 12.0/8368 | Neck mass | Right thyroid, 3.5×3×2, 22 | En bloc resection | Yes, lung | Hypercalcemia | NA/NA |
| 11 [11] | 11/M | NA | NA/1630 | Bowing of legs | RIPA, NA, NA | Simple excision, then hemithyroidectomy | No | Normocalcemia | NA/NA |
| 12 [12] | 10/F | NA | 12.2/2217 | Pain abdomen, deformities | LPA, NA, NA | NA | NA | NA | NA/NA |
| 13 [13] | 8/F | No | 12.5/453 | Pathological fracture, renal calculi, weight loss, fatigue | Multiple neck masses, NA, NA | Simple excision (n = 3), en bloc resection (n = 1) | Yes, neck nodules, lung | Hypercalcemia | NA/heterozygous partial deletion of CDC73 |
| 14b | 16/M | No | 15.6/2028 | Nonhealing fracture, neck mass, short stature, calculi | RIPA, 3×3×2.5, 10 | En bloc resection | No | Normocalcemia | Sparse parafibromin nuclear staining/heterozygous deletion of CDC73 |
Abbreviations: FH, family history; IHC, immunohistochemistry; LIPA, left inferior parathyroid adenoma; LPA, left parathyroid adenoma; LSPA: left superior parathyroid adenoma; NA, not available; RIPA, right inferior parathyroid adenoma; RSPA, right superior parathyroid adenoma.
PTH measured in Eq/mL.
Index case.
3. Discussion
The index case depicts a unique clinical scenario wherein a child had presented with a nonhealing fragility fracture and a visible neck mass. Although the biochemical diagnosis of PHPT was straightforward, the etiological diagnosis of an underlying PC was not certain prior to surgery. The pretest probability of it being a benign parathyroid adenoma was high. However, soft pointers that suggested a diagnosis of parathyroid malignancy were a visible neck mass (which was confirmed on imaging as being parathyroid in origin), surrounding thyroid infiltration, and very high serum calcium levels. Thyroid infiltration was confirmed intraoperatively, and the final diagnosis was clinched on histopathological examination.
Due to the lack of sufficient data on the entity of pediatric PC, the subsequent evidence on parathyroid cancer is mostly derived from adult case series and reviews. References to pediatric data are made wherever substantial evidence exists.
A. Prevalence
PHPT is an uncommon endocrine disease in the pediatric population, with a prevalence of two to three cases per 100,000 [1]. Causes in this age group include sporadic adenomas (up to 80%) and familial syndromes like MEN 1 and MEN 2A, HPT-JT syndrome, and familial isolated hyperparathyroidism. PC as an etiology of pediatric PHPT is rare, and no data on prevalence or sex predilection are available in the pediatric population.
B. Pathogenesis
The pathogenesis of PC remains unknown. In adults, there have been associations with irradiation [19], end-stage renal disease [20], and previous hyperplastic or adenomatous parathyroid [21]. However, Schantz and Castleman [2] reviewed 70 cases and found no evidence for malignant transformation of previously pathologic parathyroid tissue. According to the Swedish Family Cancer Database, a previous diagnosis of thyroid cancer can be considered a major risk predictor for malignant parathyroid disease [22]. Germline mutations in HRPT2 gene (located on 1q21-q32), also known as CDC73, have been found in both syndromic (HPT-JT syndrome) and sporadic (20% to 40%) parathyroid carcinomas [23]. Shattuck et al. [24] demonstrated that 10 of 15 (67%) apparently sporadic parathyroid carcinomas had HRPT2 mutations, three of which had germline mutations. Cardoso et al. [23] summarized 66 cases of sporadic parathyroid carcinomas with CDC73 mutations. Three of these cases had gross deletions, like our index case, whereas the majority of the others were either truncating mutations or mutations affecting only the start codon. There was no obvious genotype–phenotype correlation. Accordingly, genetic testing for CDC73 is recommended in every patient with PC. PC has also been described in the context of MEN 1 and MEN 2 syndromes and germline GCM2 mutations [25].
C. Presentation
PHPT in the pediatric population is almost always symptomatic irrespective of the underlying cause [1]. Renal and bone manifestations are usually present at the time of diagnosis. There is no differentiating clinical feature or distinct severity of symptom/sign for PC vs benign PHPT in this age group. Still, the presence of a palpable neck mass, a jaw tumor, and a family history of HPT-JT syndrome have been postulated as risk factors for PC. Atypical presentation in the form of nonfunctioning parathyroid carcinoma with normocalcemia has also been described [26, 27] but not in a child. PHPT is also known to present with rachitic features in children; however, a similar presentation is yet to be reported in PC [28, 29]. This has been ascribed to both nutritional vitamin D deficiency (due to anorexia, nausea, and vomiting) and renal phosphate wasting. The index case also had florid rachitic features at presentation, with both vitamin D deficiency and PHPT likely being the contributing factors.
D. Diagnosis
The usual biochemical tests (serum and urinary calcium, phosphate, alkaline phosphatase, vitamin D, iPTH, bone turnover markers) used for the diagnosis of PHPT are not robust enough to discriminate between adenoma and carcinoma. Nevertheless, there have been efforts to assess the preoperative risk of carcinoma in patients with PHPT using serum calcium, human chorionic gonadotrophin, adenoma size, ultrasonographic characteristics, and PTH assay ratio. Preoperative corrected calcium levels >3.0 mmol/L (>12.02 mg/dL) were reported in 85.3% of patients with PC in one study [30]. In an observational study, both urinary and serum human chorionic gonadotrophin (hyperglycosylated/malignant isoform only) were found to be significantly elevated in subjects with PC compared with subjects with benign PHPT [31]. Regarding the size, parathyroid carcinomas were found to be >3.0 cm in 80% of patients in one study [30] and 76.6% of patients in another report [32]. Consequently, Schulte and Talat [33] proposed the “<3 + <3” rule for exclusion of parathyroid cancer, with a positive predictive value of 99.8% in real-life populations. In a retrospective study of 16 parathyroid carcinomas, the presence of irregular margins, inhomogeneous echogenicity, and a depth/width ratio ≥1 were found to be predictive of carcinoma [34]. In another study, the presence of infiltration and calcification predicted PC, with a positive predictive value of 100% [35]. Despite this evidence, there are no concrete recommendations to advocate the use of ultrasonography or that of other imaging modalities (CT, 11C-methionine-PET, 99mTc-MIBI SPECT) in patients with PC. We prefer a cervico-thoracic CT to gauge the local invasiveness and extent of the lesion. Three studies have explored the differences in the measurement of PTH using second- and third-generation assays [36–38]. These authors have concluded that the PTH ratio (third-generation to second-generation assay) of >1.0 has a high positive predictive value for a preoperative diagnosis of PC.
E. Treatment
As in adults, surgery is the most effective and possibly curative therapy for pediatric patients with PC. The choice of surgical procedure is usually guided by the preoperative suspicion, local extent of involvement, and intraoperative findings. Parathyroid adenomas are usually locally excised. However, the latter is associated with an increased risk of positive surgical margins along with single or multiple repeat surgeries in patients with parathyroid carcinomas [30]. For PC, en bloc excision of ipsilateral thyroid lobe and isthmus with central-cervical lymph node compartments is recommended [33]. Some researchers prefer resection of the involved recurrent laryngeal nerve if the tumor cannot be dissected from the nerve completely [39]. En bloc/radical excision has been shown to be independently associated with decreased PC recurrence [40]. In spite of this knowledge, most patients undergo simple excision, which reflects the diagnostic challenges prior to initial surgery. If a frozen section shows positive resection margins, a parallel intraoperative assessment of PTH and calcium levels can identify persistent or residual disease. Persistent disease should be located and, when possible, cleared surgically followed by adjuvant radiotherapy. In patients without PTH excess, positive resection margins still predict a high risk of local recurrence, and such individuals need close follow-up every 3 to 6 months for 5 to 10 years [33].
F. Histopathology
The criterion of parathyroid malignancy was laid down by Schantz and Castleman [2]. This included existence of fibrous bands resulting in a lobular architecture, nuclear atypia (macronucleoli), and mitotic figures. These features are quite inconsistent; thus, histopathology is frequently questioned and challenged [41]. A definitive diagnosis of malignancy is restricted to tumors displaying evidence of vascular invasion (in the capsule or adjacent tissue), capsular invasion with growth into adjacent tissue, or metastasis [42]. A close histological differential is atypical parathyroid adenoma, but the latter lacks vascular invasion, metastases, or increased mitotic activity. A proliferation index (the Ki-67 index), evaluated by the MIB1 antibody, higher than 5% is associated with a higher risk of malignancy and recurrence [43]. Immuno-histochemical staining for protein gene product 9.5 and parafibromin (encoded by CDC73) has been used to confirm the diagnosis because carcinomas (with CDC73 mutations) are negative for these proteins [44].
G. Prognosis
Recurrence in parathyroid carcinoma is fairly common due to the propensity of loco-regional spread or distant vascular dissemination. Recurrence has been reported in 51.6% patients in the largest series [30]. Metastasis to lung (variably 40% to 80%), cervical nodes (up to 30%), bones (up to 11%), and mediastinum occurs, but late in the course of the disease [30, 45]. The mortality rate due to parathyroid cancer has been described to be 30% to 50% [30, 32].
In summary, due to the rarity of pediatric parathyroid carcinoma, the diagnosis of this endocrine neoplasm remains a challenge. Recurrent elevation of iPTH after adenoma excision, histology (both gross and microscopic), surgeon’s notes (local invasion), and preoperative evidence (severe hypercalcemia, lesion size >3 cm, ultrasound characteristics) can point toward the diagnosis. High suspicion allows for timely en bloc resection and a favorable outcome.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- HPT-JT
hyperparathyroidism-jaw tumor
- iPTH
intact parathyroid hormone
- MEN
multiple endocrine neoplasia
- PC
parathyroid carcinoma
- PHPT
primary hyperparathyroidism
Contributor Information
Márta Korbonits, Email: marta.korbonits@nhs.net.
Sanjay Kumar Bhadada, Email: bhadadask@rediffmail.com.
References and Notes
- 1. Roizen J, Levine MA. Primary hyperparathyroidism in children and adolescents. J Chin Med Assoc. 2012;75(9):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schantz A, Castleman B. Parathyroid carcinoma: a study of 70 cases. Cancer. 1973;31(3):600–605. [DOI] [PubMed] [Google Scholar]
- 3. Fujimoto Y, Obara T, Ito Y, Kanazawa K, Aiyoshi Y, Nobori M. Surgical treatment of ten cases of parathyroid carcinoma: importance of an initial en bloc tumor resection. World J Surg. 1984;8(3):392–400. [DOI] [PubMed] [Google Scholar]
- 4. Young TO, Saltzstein EC, Boman DA. Parathyroid carcinoma in a child: unusual presentation with seizures. J Pediatr Surg. 1984;19(2):194–196. [DOI] [PubMed] [Google Scholar]
- 5. McHenry CR, Rosen IB, Walfish PG, Cooter N. Parathyroid crisis of unusual features in a child. Cancer. 1993;71(5):1923–1927. [DOI] [PubMed] [Google Scholar]
- 6. Meier DE, Snyder WH III, Dickson BA, Margraf LR, Guzzetta PC, Jr. Parathyroid carcinoma in a child. J Pediatr Surg. 1999;34(4):606–608. [DOI] [PubMed] [Google Scholar]
- 7. Hamill J, Maoate K, Beasley SW, Corbett R, Evans J. Familial parathyroid carcinoma in a child. J Paediatr Child Health. 2002;38(3):314–317. [DOI] [PubMed] [Google Scholar]
- 8. Fiedler AG, Rossi C, Gingalewski CA. Parathyroid carcinoma in a child: an unusual case of an ectopically located malignant parathyroid gland with tumor invading the thymus. J Pediatr Surg. 2009;44(8):1649–1652. [DOI] [PubMed] [Google Scholar]
- 9. Herrera-Hernández AA, Aranda-Valderrama P, Díaz-Pérez JA, Herrera LP. Intrathyroidal parathyroid carcinoma in a pediatric patient. Pediatr Surg Int. 2011;27(12):1361–1365. [DOI] [PubMed] [Google Scholar]
- 10. Kim YS. Parathyroid carcinoma with lung metastasis in a thirteen-year-old girl. J Korean Surg Soc. 2012;82(6):385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vinodh M, Rajeshwari A. Parathyroid carcinoma presenting as genu valgum. Indian Pediatr. 2012;49(2):156. [DOI] [PubMed] [Google Scholar]
- 12. Rahman MM, Karim SS, Joarder AI, Mubin S, Abir MM, Morshed MS. Parathyroid carcinoma in a 10 years old female child. Mymensingh Med J. 2015;24(3):619–623. [PubMed] [Google Scholar]
- 13. Davidson JT, Lam CG, McGee RB, Bahrami A, Diaz-Thomas A. Parathyroid cancer in the pediatric patient. J Pediatr Hematol Oncol. 2016;38(1):32–37. [DOI] [PubMed] [Google Scholar]
- 14. Mallet E; Working Group on Calcium Metabolism. Primary hyperparathyroidism in neonates and childhood: the French experience (1984-2004). Horm Res. 2008;69(3):180–188. [DOI] [PubMed] [Google Scholar]
- 15. Kollars J, Zarroug AE, van Heerden J, Lteif A, Stavlo P, Suarez L, Moir C, Ishitani M, Rodeberg D. Primary hyperparathyroidism in pediatric patients. Pediatrics. 2005;115(4):974–980. [DOI] [PubMed] [Google Scholar]
- 16. Ruda JM, Hollenbeak CS, Stack BC, Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132(3):359–372. [DOI] [PubMed] [Google Scholar]
- 17. Belcher R, Metrailer AM, Bodenner DL, Stack BC, Jr. Characterization of hyperparathyroidism in youth and adolescents: a literature review. Int J Pediatr Otorhinolaryngol. 2013;77(3):318–322. [DOI] [PubMed] [Google Scholar]
- 18. Korpi-Hyövälti E, Cranston T, Ryhänen E, Arola J, Aittomäki K, Sane T, Thakker RV, Schalin-Jäntti C. CDC73 intragenic deletion in familial primary hyperparathyroidism associated with parathyroid carcinoma. J Clin Endocrinol Metab. 2014;99(9):3044–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christmas TJ, Chapple CR, Noble JG, Milroy EJ, Cowie AG. Hyperparathyroidism after neck irradiation. Br J Surg. 1988;75(9):873–874. [DOI] [PubMed] [Google Scholar]
- 20. Berland Y, Olmer M, Lebreuil G, Grisoli J. Parathyroid carcinoma, adenoma and hyperplasia in a case of chronic renal insufficiency on dialysis. Clin Nephrol. 1982;18(3):154–158. [PubMed] [Google Scholar]
- 21. Desch CE, Arsensis G, Woolf PD, May AG, Amatruda JM. Parathyroid hyperplasia and carcinoma within one gland. Am J Med. 1984;77(1):131–134. [DOI] [PubMed] [Google Scholar]
- 22. Fallah M, Kharazmi E, Sundquist J, Hemminki K. Nonendocrine cancers associated with benign and malignant parathyroid tumors. J Clin Endocrinol Metab. 2011;96(7):E1108–E1114. [DOI] [PubMed] [Google Scholar]
- 23. Cardoso L, Stevenson M, Thakker RV. Molecular genetics of syndromic and non-syndromic forms of parathyroid carcinoma. Hum Mutat. 2017;38(12):1621–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D, Wierman ME, Tojo K, Robbins CM, Carpten JD, Farnebo LO, Larsson C, Arnold A. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349(18):1722–1729. [DOI] [PubMed] [Google Scholar]
- 25. El Lakis M, Nockel P, Guan B, Agarwal S, Welch J, Simonds WF, Marx S, Li Y, Nilubol N, Patel D, Yang L, Merkel R, Kebebew E. Familial isolated primary hyperparathyroidism associated with germline GCM2 mutations is more aggressive and has a lesser rate of biochemical cure. Surgery. 2018;163(1):31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Messerer CL, Bugis SP, Baliski C, Wiseman SM. Normocalcemic parathyroid carcinoma: an unusual clinical presentation. World J Surg Oncol. 2006;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campennì A, Ruggeri RM, Sindoni A, Giovinazzo S, Calbo E, Ieni A, Calbo L, Tuccari G, Baldari S, Benvenga S. Parathyroid carcinoma presenting as normocalcemic hyperparathyroidism. J Bone Miner Metab. 2012;30(3):367–372. [DOI] [PubMed] [Google Scholar]
- 28. Pitukcheewanont P, Numbenjapon N, Costin G. Ectopic thymic parathyroid adenoma and vitamin D deficiency rickets: a 5-year-follow-up case report and review of literature. Bone. 2008;42(4):819–824. [DOI] [PubMed] [Google Scholar]
- 29. Shah VN, Bhadada SK, Bhansali A, Behera A, Mittal BR, Bhavin V. Influence of age and gender on presentation of symptomatic primary hyperparathyroidism. J Postgrad Med. 2012;58(2):107–111. [DOI] [PubMed] [Google Scholar]
- 30. Talat N, Schulte K-M. Clinical presentation, staging and long-term evolution of parathyroid cancer. Ann Surg Oncol. 2010;17(8):2156–2174. [DOI] [PubMed] [Google Scholar]
- 31. Rubin MR, Bilezikian JP, Birken S, Silverberg SJ. Human chorionic gonadotropin measurements in parathyroid carcinoma. Eur J Endocrinol. 2008;159(4):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hundahl SA, Fleming ID, Fremgen AM, Menck HR; The American College of Surgeons Commission on Cancer and the American Cancer Society. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1995: a National Cancer data base report. Cancer. 1999;86(3):538–544. [DOI] [PubMed] [Google Scholar]
- 33. Schulte K-M, Talat N. Diagnosis and management of parathyroid cancer. Nat Rev Endocrinol. 2012;8(10):612–622. [DOI] [PubMed] [Google Scholar]
- 34. Hara H, Igarashi A, Yano Y, Yashiro T, Ueno E, Aiyoshi Y, Ito K, Obara T. Ultrasonographic features of parathyroid carcinoma. Endocr J. 2001;48(2):213–217. [DOI] [PubMed] [Google Scholar]
- 35. Sidhu PS, Talat N, Patel P, Mulholland NJ, Schulte K-M. Ultrasound features of malignancy in the preoperative diagnosis of parathyroid cancer: a retrospective analysis of parathyroid tumours larger than 15 mm. Eur Radiol. 2011;21(9):1865–1873. [DOI] [PubMed] [Google Scholar]
- 36. Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, Simonds WF, Gillanders EM, Kennedy AM, Chen JD, Agarwal SK, Sood R, Jones MP, Moses TY, Haven C, Petillo D, Leotlela PD, Harding B, Cameron D, Pannett AA, Höög A, Heath H III, James-Newton LA, Robinson B, Zarbo RJ, Cavaco BM, Wassif W, Perrier ND, Rosen IB, Kristoffersson U, Turnpenny PD, Farnebo LO, Besser GM, Jackson CE, Morreau H, Trent JM, Thakker RV, Marx SJ, Teh BT, Larsson C, Hobbs MR. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32(4):676–680. [DOI] [PubMed] [Google Scholar]
- 37. Caron P, Maiza JC, Renaud C, Cormier C, Barres BH, Souberbielle JC. High third generation/second generation PTH ratio in a patient with parathyroid carcinoma: clinical utility of third generation/second generation PTH ratio in patients with primary hyperparathyroidism. Clin Endocrinol (Oxf). 2009;70(4):533–538. [DOI] [PubMed] [Google Scholar]
- 38. Rubin MR, Silverberg SJ, D’Amour P, Brossard J-H, Rousseau L, Sliney J, Jr, Cantor T, Bilezikian JP. An N-terminal molecular form of parathyroid hormone (PTH) distinct from hPTH(1 84) is overproduced in parathyroid carcinoma. Clin Chem. 2007;53(8):1470–1476. [DOI] [PubMed] [Google Scholar]
- 39. Adam MA, Untch BR, Olson JA, Jr. Parathyroid carcinoma: current understanding and new insights into gene expression and intraoperative parathyroid hormone kinetics. Oncologist. 2010;15(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang P, Xue S, Wang S, Lv Z, Meng X, Wang G, Meng W, Liu J, Chen G. Clinical characteristics and treatment outcomes of parathyroid carcinoma: a retrospective review of 234 cases. Oncol Lett. 2017;14(6):7276–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodriguez C, Nadéri S, Hans C, Badoual C. Parathyroid carcinoma: a difficult histological diagnosis. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129(3):157–159. [DOI] [PubMed] [Google Scholar]
- 42. Roth SI. Pathology of the parathyroids in hyperparathyroidism: discussion of recent advances in the anatomy and pathology of the parathyroid glands. Arch Pathol. 1962;73:495–510. [PubMed] [Google Scholar]
- 43. Delellis RA. Challenging lesions in the differential diagnosis of endocrine tumors: parathyroid carcinoma. Endocr Pathol. 2008;19(4):221–225. [DOI] [PubMed] [Google Scholar]
- 44. Howell VM, Gill A, Clarkson A, Nelson AE, Dunne R, Delbridge LW, Robinson BG, Teh BT, Gimm O, Marsh DJ. Accuracy of combined protein gene product 9.5 and parafibromin markers for immunohistochemical diagnosis of parathyroid carcinoma. J Clin Endocrinol Metab. 2009;94(2):434–441. [DOI] [PubMed] [Google Scholar]
- 45. Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab. 2001;86(2):485–493. [DOI] [PubMed] [Google Scholar]