Abstract
Background:
Treatment patterns in Parkinson’s disease (PD) have not been extensively studied for nearly two decades. Insurance claims are appropriate for such analysis.
Objective:
To understand the standard of care use of symptomatic treatments in new cases of PD and factors associated with treatment choice.
Methods:
Retrospective cohort study using claims data from the United States between 2008 and 2016. We used Kaplan–Meier methodology to estimate time to treatment start and switch or add-on therapy and Cox proportional hazards models to identify predictors.
Results:
We identified 68,532 patients eligible for treatment pattern analyses. Median time from diagnosis until first treatment was 37 days (95% confidence interval: 36–38). Two distinct patterns of treatment initiation were identified: fast initiators and patients with delayed treatment start (or no recorded treatment). Levodopa therapies were the most commonly prescribed treatment class (52.6%). Increased age was associated with shorter time to start of treatment with levodopa. Younger age was associated with shorter time to initiation of dopamine agonists and other symptomatic treatments. Patients that initiated treatment with levodopa/combinations had the fewest switches/add-ons [30.4%; median time 7.29 (6.71, 8.13) years]. Older patients had fewer switch/add-on therapies, but only in the group that started with levodopa/combination therapy.
Conclusions:
Time from diagnosis to treatment start was relatively short, suggesting that PD diagnosis, as reflected in the database, is closely linked to start of symptomatic treatment. Levodopa treatment remains the most common treatment, especially for older patients. Delayed treatment start was associated with increased age and comorbidity.
Keywords: Charlson comorbidities, claims data, Parkinson’s disease, treatment patterns, United States
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder affecting approximately one in 100 people over the age of 60 [1, 2]. Although motor symptoms are central to PD, it is now recognized that non-motor abnormalities including sleep behavior disorders, olfactory dysfunction, constipation, excessive daytime somnolence, hypotension, erectile dysfunction, urinary dysfunction, and depression can also be symptoms of PD and may be present prior to diagnosis of PD, which is based on motor symptoms [3].
Since its introduction in the 1960 s, levodopa has been the gold-standard choice for treatment of PD-related motor symptoms, such as slowness of movement, rigidity and tremor [4]. Levodopa loses efficacy over time and prolonged use is accompanied by motor complications including refractory motor fluctuations and dyskinesia, which are observed in almost all patients with PD after 4–6 years [5–9]. Despite the availability of other symptomatic treatments such as dopamine agonists, monoamine oxidase type B inhibitors (MAO-Bi) and amantadine—used instead of, or in combination with, levodopa in order to optimize symptomatic control—an unmet medical need still remains, as no treatments with a direct effect on the underlying disease pathophysiology are currently available and the disease continues to progress regardless of symptomatic therapy [5, 10, 11].
It is important to understand current standard of care (SOC) in PD in detail to identify appropriate comparators for new investigational medications and to monitor if current guidelines are being followed. Recent treatment guidelines and reviews covering therapeutic management of early PD include the National Institute for Health and Care Excellence (NICE) and Scottish Intercollegiate Guidelines Network (SIGN) guidelines in the United Kingdom [12, 13]; a report of the European Federation of Neurological Societies (EFNS)/Movement Disorder Society (MDS) [14] and the American Academy of Neurology (AAN) practice parameter report [15] and other country-specific guidelines. It is generally recommended to initiate treatment when motor symptoms start to have an impact on the patient’s life, although the optimal time frame for initiation of therapy has not been clearly defined. For example, guidelines also suggest considering a combination of factors including relative effectiveness for symptomatic control/prevention of motor complications and adverse effect profile of the drugs, patient symptoms, age, needs and expectations, experience, comorbidities, environment and employment status. The choice should be made following individual assessment and discussion and the timing of when to start treatment is determined by the patient’s individual circumstances [13, 14].
In a recent review Bloem et al. [16] stressed the importance of large, routinely collected, administrative insurance claims databases as an opportunity to study large numbers of PD patients, in settings reflective of ‘real-life’ clinical practice. Importantly, a study by Willis et al. using insurance claims data from the United States (US) Medicare program found that neurologist care of patients with PD may be associated with improved selected clinical outcomes and greater survival [17]. However, of the 31 such studies Bloem et al. examined, just one [18] studied treatment patterns, and with a particular focus on costs and adverse events, rather than treatment patterns per se. To our knowledge, only one other peer reviewed manuscript with such an objective has been published to date, and this was based on data from almost two decades ago [19].
In an attempt to fill this gap, we identified a large incident cohort of PD patients from insurance claims data and used this to understand current SOC patterns of use of symptomatic treatments for motor symptoms of PD. Specifically we were interested in the timing of treatment initiation relative to diagnosis. We also studied the timing of switching and/or adding other therapies and how these patterns vary with respect to age, gender and comorbidity burden.
METHODS
Data source and setting
This was a retrospective observational study set in the US. We utilized data from the Truven Health MarketScan® Commercial Claims and Medicare Supplemental databases. Established in the early 1990 s, the MarketScan databases contain individual-level, de-identified, healthcare claims across inpatient, outpatient, prescription drug, and carveout services. The strengths of the MarketScan databases lie in their large sample size, completion of episodes of care in different settings, and strong longitudinal tracking at the patient level.
The Commercial Claims (‘commercial’) data contains active employees, early retirees and dependents insured by employer-sponsored plans. The Medicare Supplemental (‘Medicare’) data covers Medicare-eligible retirees (≥65 years) with employer-sponsored Medicare Supplemental plans. It is possible to track patients from employment into retirement between the Commercial claims and the Medicare claims portions of MarketScan. Therefore, these two parts of the MarketScan data were analyzed together. These data are fully anonymized and comply with the US Health Insurance Portability and Accountability Act (HIPAA).
Study design and cohort selection
The study cohort of interest was newly diagnosed PD patients. We identified PD patients via International Classification of Disease (ICD) codes. Specifically, we required ≥2 occurrences of ICD9 332.0 and/or ICD10 G20 codes, at least 1 day apart. We ensured subjects had full enrollment in the database and no PD code for at least 12 months beforehand, in order to capture incident cases and to derive baseline comorbidities (see ‘variables’ section). Similar strategies with administrative data have been successfully used in previous PD research [20, 21].
The main study period was from 1 January 2008 to 31 December 2016. Patients were followed from the date of first PD diagnosis claim until discontinuation from the database (for any reason, e.g., changed insurance or death, etc.) or until the end of analysis period (31 December 2016); whichever occurred first. Short gaps in insurance enrollment of ≤31 days were allowed, so long as they were surrounded by enrollment periods both before and afterwards. The rationale for this was to allow small periods for delayed administration or insurance payments. Only patients with drug coverage (as well as medical coverage) on the date of PD diagnosis (first claim) were considered for the treatment pattern analyses.
Variables
In order to account for burden of disease and comorbidities, we derived Charlson Comorbidities and the Charlson Comorbidity Index (CCI) for each patient [22]. We based our code lists on updated versions which were revised specifically for ICD9 and ICD10 in insurance claims data [23, 24]. We used the time frame of between 12 months and 1 month prior to PD diagnosis to screen for the presence/absence of each of the 17 comorbidities. The CCI was calculated as the weighted sum of present comorbidities. Higher weights are given to the more severe comorbidities so high CCI represents greater comorbidity burden (see Supplementary Table 2).
Treatments identified mainly represent mail-order and card program outpatient pharmaceutical drug claim prescriptions. They were identified via National Drug Codes and grouped into the following five classes: levodopa and levodopa combination therapies with carbidopa and/or entacapone (hereafter “levodopa/combination”), dopamine agonists (cabergoline; ropinirole; pramipexole; bromocriptine; pergolide; apomorphine and rotigotine), MAO-Bi (selegiline and rasagiline), amantadine and “other dopaminergic agents” (the Catechol-O-methyl transferase (COMT) inhibitors entacapone and tolcapone). Apomorphine was also identified by the Healthcare Common Procedure Coding System (HCPCS) code J0364 from in-/out-patient hospital settings.
Statistical analysis
Cohort demographics, Charlson comorbidities and most common initial treatment classes were descriptively analyzed. For treatments, this included combinations of classes when two or more were prescribed on the same day.
For analysis regarding time to initiation of specific treatment classes, ‘events’ and event times were defined based upon time between PD diagnosis and first dispense of the treatment class in question. This was regardless of whether or not the treatment class was received as the first treatment or subsequently. Only patients that received an initial treatment were eligible for the time to switch/add-on analyses. We did not require that patients receive their initial medication continuously, so the time to switch/add-on was calculated as the number of days between dispense of the initial treatment class and second treatment class different to the first, regardless of whether they discontinued the first or not.
For patients treated with levodopa/combination at any time after PD diagnosis, we considered discontinuation as a gap of more than 180 days from the last supply-day of their previous prescription or no new levodopa/combination prescription at all. If patients discontinued from the database before a 180-day gap, their data was censored on that day.
Patient time was always censored for those without an event upon discontinuation from the database (for any reason) or at the end of study follow-up, whichever came first. The time until first treatment, first switch/add-on and levodopa/combination discontinuation were all calculated and plotted using Kaplan–Meier methodology. We also fitted Cox proportional hazards models to describe the associations between treatment initiation and switch/add-on, overall and by treatment class. Explanatory variables included were patient age at diagnosis, gender and CCI score. We additionally included time to first treatment as an explanatory variable in the time to switch/add-on analysis.
Sensitivity analysis
Post hoc sensitivity analyses were performed to include year of first PD claim, region (based on US census region) and specialty of physician at first diagnosis claim for PD as covariates in Cox regression analyses of treatment initiation and switch/add-on.
RESULTS
Patient population
A total of 209,046 subjects were identified with at least two PD claims across the whole database. Of these, 84,104 could be identified as incident cases; and thus were included into the study cohort (see Supplementary Table 1).
Table 1 displays the characteristics of the cohort. The mean (standard deviation [SD]) age at first PD diagnosis was 73.4 (12.0) years and 58.3% of the cohort were male. Only a minority of patients were identified prior to 50 years of age [n = 2,508; 3.0% ]. All regions of the US were well represented in terms of raw numbers. The mean (SD) follow-up time was 5.9 (2.6) years across the whole database and 2.3 (1.9) years after diagnosis.
Table 1.
Characteristics of newly diagnosed Parkinson’s disease cohort (n = 84,104)
| n | % | |
| Gender | ||
| Female | 35,060 | 41.69 |
| Male | 49,044 | 58.31 |
| Age | ||
| Mean (SD) [median] | 73.4 (12.0) [75.0] | |
| <50 | 2,508 | 2.98 |
| 50–70 | 28,670 | 34.09 |
| >70 | 52,926 | 62.93 |
| Region | ||
| North East | 16,248 | 19.32 |
| North Central | 25,962 | 30.87 |
| South | 25,153 | 29.91 |
| West | 15,319 | 18.21 |
| Unknown | 1,422 | 1.69 |
| Year of First PD Claim | ||
| 2008–2010 | 30,482 | 36.24 |
| 2011–2013 | 31,702 | 37.69 |
| 2014–2016 | 21,920 | 26.06 |
| Specialty of First PD Claim | ||
| Neurologist | 33,617 | 39.97 |
| Other | 50,487 | 60.03 |
| Length of follow-up (years) | ||
| From database enrollment [mean (SD)] | 5.9 (2.6) | |
| From PD diagnosis [mean (SD)] | 2.3 (1.9) |
Regions defined as per US census. Total database enrollment time includes 1 year ‘disease-free’ period for each patient. PD, Parkinson’s disease; SD, standard deviation.
Out of the full PD cohort, 68,532 (81.5%) patients had insurance drug coverage (as well as medical) and were therefore eligible for treatment pattern analyses.
Charlson comorbidities
Supplementary Table 2 displays the frequencies of Charlson comorbidities overall and by age group. Almost two-thirds [n = 54,410; 64.7% ] of patients had at least one comorbid condition and 28.7% had a CCI score of 3 or more.
The most prevalent comorbidities were diabetes mellitus [n = 20,829, 24.8% without complications and n = 7,357; 8.7% with complications], cerebrovascular disease [n = 18,370; 21.8% ], and congestive heart disease [n = 14,797; 17.6% ]. Other serious comorbid diseases with high prevalence were peripheral vascular disease [n = 12,454; 14.8% ], chronic pulmonary disease [n = 10,171; 12.1% ] and cancer [n = 10,163; 12.1% ].
The proportion of patients with at least one comorbidity increased with age from 31.8% for those <50 years, to 51.1% for 50–70 years, and finally to 73.6% for those >70 years old. The mean (and median) CCI score also increased with age from 0.66 (0) in those <50 years to 1.31 (1) for 50–70 years, to 2.20 (2) in those >70 years. The majority of individual comorbidities also markedly increased in prevalence with age.
Treatment initiation
Table 2 shows the most commonly used first-line treatments. Of the 49,737 (72.6%) patients whose first treatment was observed during follow-up, 70.3% were prescribed levodopa/combination therapy, making it by far the most commonly used treatment class. The second most frequently prescribed treatment class was dopamine agonists [n = 7,729; 15.5% ] and 1% of patients started with both levodopa/combination and dopamine agonists on the same day.
Table 2.
First treatment after Parkinson’s disease diagnosis (n = 68,532)
| First treatment class | Treated | Patients eligible for treatment pattern analysis (n = 68,532)* | Patients treated (n = 49,737) | ||||
| Amantadine | Levodopa and levodopa combinations | Dopamine agonists | MAO-Bi | Other dopaminergic agents | n | % | % |
| x | 34,977 | 51.0 | 70.3 | ||||
| x | 7,729 | 11.3 | 15.5 | ||||
| x | 4,112 | 6.0 | 8.3 | ||||
| x | 1,521 | 2.2 | 3.1 | ||||
| x | x | 521 | 0.8 | 1.0 | |||
| x | x | 234 | 0.3 | 0.5 | |||
| x | x | 148 | 0.2 | 0.3 | |||
| x | x | 132 | 0.2 | 0.3 | |||
| x | x | 106 | 0.2 | 0.2 | |||
| x | 100 | 0.1 | 0.2 | ||||
| Other combinations+ | 157 | 0.2 | 0.3 | ||||
Multiple “x”s in one row indicate that two or more treatments from different classes were dispensed on the same day. *These patients met overall inclusion/exclusion criteria for treatment pattern analyses (see Supplementary Table 1). +The full list of infrequently prescribed combinations has been summarized in one row. MAO-Bi, monoamine oxidase type B inhibitors.
First-line use of monotherapy MAO-Bi was reported in 8.3% of patients and first-line use of amantadine was 3.1%.
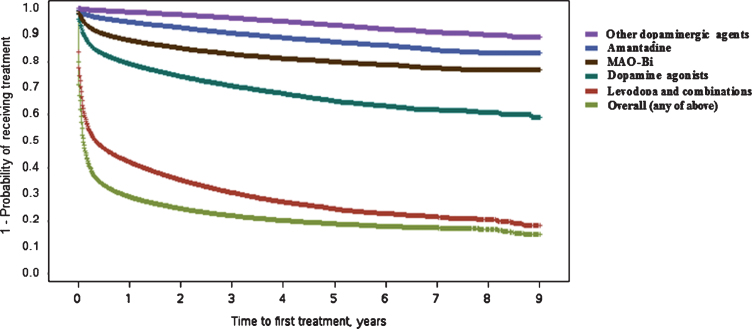
Overall, the median time to treatment start was 37 days (95% confidence interval [CI]: 36–38). The Kaplan–Meier plot for time to initiation of any treatment (Fig. 1) suggested two distinct groups of patients, namely: fast initiators and those with more delayed treatment start (or no recorded treatment). One-quarter of patients filled a prescription within 3 days of first PD diagnosis code. Roughly two-thirds (66.3%) were treated within the first 6 months, and 70.8% were treated by the end of Year 1, which can be seen by the sharp drop in the Kaplan–Meier curve until around this time. Treatment rate estimates later in follow-up were based on more limited numbers of patients who had not already been treated or discontinued from the database. For example, after 3 years of follow-up, 3,732 patients were still enrolled in the database with no recorded first treatment. Only 99 patients were still enrolled with no recorded treatment after 8 years of follow-up. Within the group of patients with no recorded treatment during at least 3 years of follow-up after diagnosis, 71% had at least three claims for PD and 41% had at least six claims for PD.
Fig.1.
Kaplan–Meier plot for time (in years) between Parkinson’s disease diagnosis and first treatment with different drug classes. Curves represent time to first treatment with each treatment class, after PD diagnosis. Curves are not mutually exclusive. MAO-Bi, monoamine oxidase type B inhibitors; PD, Parkinson’s disease.
For any treatment, the youngest age group (<50 years) were least likely to initiate treatment early [50–70 vs <50 hazard ratio (HR): 1.37 (1.30–1.45); >70 vs <50 HR: 1.21 (1.14–1.28), see Table 3]. Higher comorbidity burden was associated with delayed treatment start [from; CCI 1 vs 0 HR: 0.94 (0.91–0.96) to; CCI 3+ vs 0 HR: 0.79 (0.77–0.80)]. Females on average, had a more delayed treatment start [HR: 0.93 (0.92–0.95)]. The time to initiation of ‘any treatment’ analysis, was mainly driven by the levodopa/combination treatments, seeing as the majority of patients initiated with this class.
Table 3.
Cox-proportional hazards models for time to first treatment and switch/add-on
| Hazard ratios for time between PD diagnosis and first treatment with: | |||||||
| Any treatment | Levodopa/combination | Dopamine agonists | MAO-Bi | Amantadine | Other dopaminergic agent | ||
| Gender | Female | 0.93 (0.92, 0.95) | 0.91 (0.90, 0.93) | 0.99 (0.96, 1.02) | 0.81 (0.78, 0.84) | 0.99 (0.94, 1.05) | 0.78 (0.72, 0.86) |
| Age, years* | 50–70 | 1.37 (1.30, 1.45) | 1.55 (1.45, 1.66) | 0.93 (0.86, 0.99) | 0.89 (0.82, 0.97) | 0.78 (0.69, 0.88) | 0.83 (0.68, 1.03) |
| >70 | 1.21 (1.14, 1.28) | 1.91 (1.79, 2.04) | 0.39 (0.36, 0.42) | 0.30 (0.28, 0.33) | 0.33 (0.30, 0.38) | 0.62 (0.51, 0.77) | |
| CCI$ | 1 | 0.94 (0.91, 0.96) | 0.98 (0.96, 1.01) | 0.83 (0.80, 0.86) | 0.70 (0.66, 0.74) | 0.84 (0.78, 0.90) | 0.80 (0.72, 0.90) |
| 2 | 0.88 (0.86, 0.91) | 0.93 (0.90, 0.96) | 0.78 (0.74, 0.82) | 0.63 (0.59, 0.67) | 0.78 (0.71, 0.85) | 0.70 (0.61, 0.80) | |
| 3+ | 0.79 (0.77, 0.81) | 0.86 (0.84, 0.88) | 0.66 (0.63, 0.69) | 0.41 (0.38, 0.43) | 0.66 (0.61, 0.71) | 0.62 (0.55, 0.70) | |
| Hazard ratios for time to switch/add-on of another treatment class, following first-line treatment with: | |||||||
| Any treatment | Levodopa/combination | Dopamine agonists | MAO-Bi | Amantadine | Other dopaminergic agent | ||
| Gender | Female | 0.92 (0.90, 0.95) | 0.93 (0.90, 0.97) | 0.85 (0.81, 0.90) | 0.95 (0.88, 1.02) | 0.92 (0.82, 1.03) | 0.98 (0.74, 1.31) |
| Age, years* | 50–70 | 0.82 (0.76, 0.88) | 0.74 (0.66, 0.83) | 0.97 (0.86, 1.09) | 0.96 (0.83, 1.10) | 1.10 (0.85, 1.42) | 0.88 (0.12, 6.35) |
| >70 | 0.40 (0.37, 0.42) | 0.36 (0.32, 0.41) | 0.88 (0.77, 0.99) | 0.91 (0.79, 1.06) | 1.03 (0.79, 1.35) | 0.83 (0.11, 5.97) | |
| CCI$ | 1 | 0.81 (0.78, 0.84) | 0.83 (0.79, 0.87) | 0.83 (0.77, 0.89) | 0.90 (0.82, 0.99) | 0.82 (0.71, 0.95) | 0.70 (0.48, 1.00) |
| 2 | 0.76 (0.73, 0.80) | 0.80 (0.75, 0.85) | 0.75 (0.69, 0.82) | 0.93 (0.83, 1.04) | 0.68 (0.57, 0.82) | 0.83 (0.54, 1.27) | |
| 3+ | 0.65 (0.62, 0.67) | 0.70 (0.66, 0.73) | 0.65 (0.60, 0.70) | 0.84 (0.75, 0.95) | 0.62 (0.52, 0.73) | 0.76 (0.54, 1.09) | |
| Time to first treatment start, in years | 0.83 (0.80, 0.86) | 0.81 (0.77, 0.85) | 0.79 (0.73, 0.84) | 0.82 (0.76, 0.90) | 0.69 (0.61, 0.79) | 0.85 (0.63, 1.14) | |
*Reference category for age is <50 years. $CCI: with reference category of CCI = 0. ‘Switch/add-on’ means the initiation of another treatment class (those listed) different to the first (regardless if first line is discontinued or not). CCI, Charlson Comorbidity Index; PD, Parkinson’s disease.
When investigating time to initiation of each individual treatment, a largely similar pattern was observed; with both female gender and higher comorbidity burden being associated with delayed onset of treatment (Table 3). However, clear differences emerged with age. While older patients were more likely to receive levodopa [>70 vs <50 HR: 1.91 (1.79–2.04)], older age was consistently and monotonically associated with a lower likelihood of receiving a dopamine agonist [>70 vs <50 HR: 0.39 (0.36–0.42)], MAO-Bi [>70 vs <50 HR: 0.30 (0.28–0.33)], amantadine [>70 vs <50 HR: 0.33 (0.30–0.38)] and other dopaminergic treatments [>70 vs <50 HR: 0.62 (0.51–0.77)].
Switch/add-on therapy
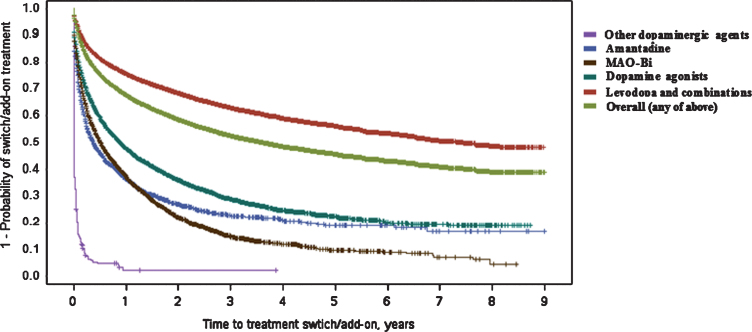
Overall, of 49,737 patients who had a first-line treatment observed, 39.1% also switched or added a second therapy during follow-up. Patients that initiated treatment with levodopa/combinations had the fewest switches/add-ons [n = 10,950; 30.4% ] while this was considerably higher for all other initial treatment classes [dopamine agonists: 60.6%; MAO-Bi: 72.8%; amantadine: 68.0% ].
In line with the above finding, the median time to switch/add-on therapy was far longer in the group that initiated treatment with levodopa/combination [7.29 (6.71–8.13) years, see Fig. 2] versus those whose first-line treatment contained a dopamine agonist [0.88 (0.83–0.94) years], MAO-Bi [0.51 (0.47–0.54) years] or amantadine [0.37 (0.31–0.43) years]. The median time to switch/add-on from the initial ‘other dopaminergic treatment’ (COMT inhibitors) group was only 1 day and the majority of these patients added levodopa as expected (92.4%; COMT inhibitors are prescribed together with levodopa and presumably these patients filled the COMT prescription before the levodopa prescription).
Fig.2.
Kaplan–Meier plot for time (in years) between first Parkinson’s disease treatment and switch/add-on of additional treatment class. ‘Switch/add-on’ means the initiation of another treatment class (those listed) different to the first (regardless if first class is discontinued or not) MAO-Bi, monoamine oxidase type B inhibitors.
Overall, and similarly to initiation of first-line treatment, female gender was associated with a longer time to switch/add-on therapy [HR: 0.92 (0.90–0.95)]. This was consistent across each of the initial treatment classes, although only statistically significant in the groups with the largest sample sizes (first-line levodopa/combination or first-line dopamine agonist groups).
Increased comorbidity burden was also associated with delayed switch/add-on therapy. This can be summarized with results from the full first-line treated cohort as results in specific treatment groups are broadly similar: [CCI 1 vs 0 HR: 0.81 (0.78–0.84); CCI 2 vs 0 HR: 0.76 (0.73–0.80); CCI 3+ vs 0 HR: 0.65 (0.62–0.67)].
For the group that initiated treatment with levodopa/combination therapy, increased age significantly lowered the likelihood of switch/add-on therapies [50–70 vs <50 HR: 0.74 (0.66–0.83);>70 vs <50 HR: 0.36 (0.32–0.41)]. A similar trend was observed in the first-line dopamine agonist- and MAO-Bi-treated groups [dopamine agonist: >70 vs <50 HR: 0.88 (0.77–0.99); MAO-Bi: >70 vs <50 HR: 0.91 (0.79–1.06)]. For the group of patients starting PD treatment with amantadine, no age versus time-to-switch/add-on relationships were found. Limited sample size made the results for the “other dopaminergic agents” group uninterpretable.
Finally, and across all initial treatment groups, longer time to initial treatment start was associated with longer time to treatment switch/add-on.
Sensitivity analysis
Overall, no obvious trend of year of first PD claim on treatment initiation was observed (Supplementary Table 3). In contrast, patients diagnosed with PD in more recent years had a lower chance of treatment switch/add-on. Including year of first PD claim as a covariate did not qualitatively impact interpretation of the pre-specified analysis with age, gender and CCI in the model.
When region was included as a covariate, no obvious trend on treatment initiation was observed overall (Supplementary Table 4). However, compared with patients in Northeast, patients in other areas were more likely to start dopamine agonists early, whereas patients in north central and south were less likely to start MAO-Bi early. Adding region did not qualitatively impact interpretation of the pre-specified analysis with age, gender and CCI in the model.
Patients whose first PD claim was reported by a non-neurologist were less likely to start treatment or switch to/add another treatment (Supplementary Table 5). Including specialty of physician at first PD claim as a covariate did not qualitatively impact interpretation of the pre-specified analysis with age, gender and CCI in the model.
Discontinuation of levodopa/combination
The median time to discontinuation of levodopa/combination therapy was 5.97 (5.72–6.25) years. This is irrespective of whether levodopa/combination was the first treatment or if other PD treatments were also used concomitantly.
DISCUSSION
In the present analysis of PD treatment patterns in the US using recent (2008–2016) data from the large Truven Health MarketScan® Commercial Claims and Medicare Supplemental databases, levodopa and levodopa combination therapies were the most commonly prescribed treatments (52.6%), followed by dopamine agonists (12.4%) and MAO-Bi (6.7%). Overall, the median time between diagnosis and initiation of treatment was relatively short [37 days 95% CI: (36–38)]. Although there may be some uncertainty in the exact date of first PD diagnosis (see limitations), the short time to initiation of treatment suggests that in the majority of cases, PD diagnoses as reflected in the claims database are closely linked to start of symptomatic treatment. However, two distinct groups of patients were observed: fast initiators and those who postponed the use of symptomatic treatment options or did not use them at all during the follow-up period. The latter group, may be partially a true reflection of a patient/physician choice to defer levodopa treatment in some patients with symptoms that do not yet severely impact function and quality of life, and with the hope to defer dyskinesia and ‘save’ the potential benefit-window for later stages of the disease when symptoms inevitably worsen [25]. On the other hand, some of these patients may have been included in our analysis due to coding error or misdiagnosis, that was subsequently corrected (e.g. essential tremor) and did not lead to prescription of the PD treatments observed in this study. A sizable proportion of these patients had three or more claims during follow-up suggesting a confirmed PD diagnosis. Therefore, they may represent a true group with delayed treatment due to slow progression or relatively early diagnosis. In addition, some of these patients may have received treatments not considered in this study.
While older patients in our study were indeed more likely to receive levodopa [>70 vs <50 HR: 1.91 (1.79–2.04)], older age was consistently and monotonically associated with a lower likelihood of dopamine agonists and other treatment classes. This finding is in line with literature stating dopamine agonists and other treatment classes should be favored in those <50 years due to their apparent higher risk of motor complications [26, 27]. The slightly delayed onset of all treatment classes we found in females can probably be explained by slightly later diagnosis in males compared with females [28]. Finally, increased comorbidity burden was consistently associated with a delayed time to any treatment start, presumably due to treatment for other conditions taking priority and/or a general decreased ability and willingness for such patients to tolerate PD medication.
In analyses adjusted for age, gender, comorbidity burden and time to first treatment, the time to treatment switch or add-on therapies was by far longest in those patients who started with levodopa/combinations (approximately 7 years) compared with those who started with levodopa-sparing treatments (all <1 year). This supports previous evidence that levodopa is the most effective and well tolerated first-line treatment choice [29]. Similar to treatment initiation, female gender and increased comorbidity burden were associated with delayed time to treatment switches or add-on therapies. Increased patient age was significantly associated with delayed time to switches from levodopa/combinations as well as directionally associated with delayed switches from dopamine agonists and MAO-Bi treatment. The median time to discontinuation of levodopa/combination therapies (in any treatment line) was roughly 6 years.
Post-hoc investigations on the association between region, year of first PD claim, and specialty of treating physician suggest these factors are also associated with treatment patterns and should be considered for more detailed future research. Importantly, their addition did not impact interpretation of the pre-specified analysis with age, gender and CCI in our study suggesting that they are independent risk factors.
Huse et al. [19] conducted the prior study most similar to ours (US claims data, median age 75) using data from 1999 to 2001. They found that within treatment users, levodopa was prescribed to the majority of patients as the initial PD medication (61.5% vs. 70.3% in the present study). Likewise, dopamine agonists were the first-line medication for 8.2% and 15.5% of patients in these studies, respectively. Overall this suggests that prescribing patterns in the US have not changed dramatically over the last two decades. Small discrepancies may be due to the fact Huse et al. counted initial prescription followed by additional other prescriptions within 30 days as combination therapy. Huse et al. also showed that older patients and those with greater comorbidity burden were more likely to start treatment with levodopa and less likely to switch thereafter.
Limitations
The major limitation of this study surrounds the unknown accuracy of identifying truly incident PD patients and the specific timing of diagnosis via insurance claims. Claims data are collected for billing purposes, so despite careful study design the diagnostic information retrieved does not necessarily reflect the first or final diagnoses for all patients. The first PD diagnosis code may represent symptoms or referrals to specialists rather than a formal diagnosis, and in any case the severity of disease at diagnosis cannot be established. A recent study created an algorithm based on 536 diagnosis codes in claims that has been able to detect truly incident PD patients with improved accuracy [30], and a similar study has been reported in electronic medical record data [31]. Furthermore, despite requiring two or more codes for PD on medical claims as part of our study inclusion criteria, it is possible we still included some misdiagnosed patients, such as has been shown in other previous secondary use databases [32]. Due to small sample sizes beyond 3 years of follow-up however, and the high proportion of such participants with further confirmatory PD claims, the absolute number of erroneously included cases should be small and their inclusion is expected to have only a negligible effect on the results of our study. The effect of excluding more misdiagnosed patients would be to further shorten our estimates of time until treatment start.
As with all insurance claims analyses, we only know the drugs were dispensed and not necessarily that the patient took them. Reasons for switching or discontinuing therapy are not documented in the database. Due to the absence of data on symptom severity and patient preferences, we cannot determine if the observed trends related to age and comorbidity burden are independent of symptom severity and patient preferences. Additionally, data on drug dosage were not included in the analyses conducted. A final limitation is that reason for database discontinuation (e.g. death, change of insurance providers and other reasons for loss to follow-up) are not well reported in the database. We were therefore unable to account for death as a competing risk in time to event analyses.
Generalizability
The study population is representative of employees, retired employees and dependents of those employed by medium- to large-sized companies in the US that offer employer-sponsored healthcare coverage. In general, therefore they are expected to be of higher socio-economic status than the general US population.
As a whole, PD patients in the cohort studied had an average onset age of 73 years. This is roughly 10 years older than the mean age of PD diagnosis reported elsewhere [1, 33], although more recent estimates are somewhat closer to our study population [34].
Conclusion
Overall, the time from diagnosis to initiation of treatment was relatively short, suggesting that claims for PD diagnosis are closely linked to claims for PD treatment in the analyzed data set. Levodopa and levodopa combination therapies remain the most commonly prescribed treatments in the US, but increased patient age and comorbidity burden play a role in the choice of treatment class prescribed and the timing of initiation relative to diagnosis. Patients initiating treatment with levodopa or levodopa combination therapy switched to or added-on other symptomatic therapy classes far later than those with a different first-line treatment.
CONFLICTS OF INTEREST
RH, FB, LV, RF, NC and RO are (or were at the time of study conduct) employed by F. Hoffmann-La Roche Ltd. YD is employed as a consultant to F. Hoffmann-La Roche Ltd. FB owns F. Hoffmann-La Roche Ltd. non-voting shares and options. F. Hoffmann-La Roche Ltd has an investigational drug for Parkinson’s disease in development and is Marketing Authorisation Holder of MADOPAR (Levodopa + Benserazide).
TRADEMARK NOTE
MarketScan is a registered trademark of Truven Health Analytics Inc., an IBM Company.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank David Evans (F. Hoffmann-La Roche Ltd.) for comments on previous draft versions of this manuscript. Study sponsored by F. Hoffmann-La Roche Ltd.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JPD-191636.
REFERENCES
- [1]. de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5, 525–535. [DOI] [PubMed] [Google Scholar]
- [2]. Nussbaum RL, Ellis CE (2003) Alzheimer’s disease and Parkinson’s disease. N Engl J Med 348, 1356–1364. [DOI] [PubMed] [Google Scholar]
- [3]. Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday G, Joseph L, Lang AE, Liepelt-Scarfone I, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G (2015) MDS research criteria for prodromal Parkinson’s disease. Mov Disord 30, 1600–1611. [DOI] [PubMed] [Google Scholar]
- [4]. Smith Y, Wichmann T, Factor SA, DeLong MR (2012) Parkinson’s disease therapeutics: New developments and challenges since the introduction of levodopa. Neuropsychopharmacology 37, 213–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Kim K-S (2017) Toward neuroprotective treatments of Parkinson’s disease. Proc Natl Acad Sci U S A 114, 3795–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Rascol O, Perez-Lloret S, Ferreira JJ (2015) New treatments for levodopa-induced motor complications. Mov Disord 30, 1451–1460. [DOI] [PubMed] [Google Scholar]
- [7]. LeWitt PA (2008) Levodopa for the treatment of Parkinson’s disease. N Engl J Med 359, 2468–2476. [DOI] [PubMed] [Google Scholar]
- [8]. Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K; Parkinson Study Group (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351, 2498–2508. [DOI] [PubMed] [Google Scholar]
- [9]. Ahlskog JE and Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16, 448–458. [DOI] [PubMed] [Google Scholar]
- [10]. Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson’s disease. J Neurochem 139(Suppl 1), 318–324. [DOI] [PubMed] [Google Scholar]
- [11]. Jankovic J and Poewe W (2012) Therapies in Parkinson’s disease. Curr Opin Neurol 25, 433. [DOI] [PubMed] [Google Scholar]
- [12].NICE (2017) Parkinson’s disease in adults | Guidance and guidelines | NICE. National Institute for Health and Care Excellence.
- [13]. SIGN (2010) Diagnosis and pharmacological management of Parkinson’s disease. A national clinical guideline - 113. Scottish Intercollegiate Guidelines Network.
- [14]. Horstink M, Tolosa E, Bonuccelli U, Deuschl G, Friedman A, Kanovsky P, Larsen JP, Lees A, Oertel W, Poewe W, Rascol O, Sampaio C (2006) Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European Federation of Neurological Societies and the Movement Disorder Society-European Section. Part I: Early (uncomplicated) Parkinson’s disease. Eur J Neurol 13, 1170–1185. [DOI] [PubMed] [Google Scholar]
- [15]. Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE (2002) Practice parameter: Initiation of treatment for Parkinson’s disease: An evidence-based review: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 58, 11–17. [DOI] [PubMed] [Google Scholar]
- [16]. Bloem BR, Ypinga JHL, Willis A, Canning CG, Barker RA, Munneke M, De Vries NM (2018) Using medical claims analyses to understand interventions for Parkinson patients. J Parkinsons Dis 8, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA (2011) Neurologist care in Parkinson disease - A utilization, outcomes, and survival study. Neurology 77, 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Suh D-C, Pahwa R, Mallya U (2012) Treatment patterns and associated costs with Parkinson’s disease levodopa induced dyskinesia. J Neurol Sci 319, 24–31. [DOI] [PubMed] [Google Scholar]
- [19]. Huse DM, Castelli-Haley J, Orsini LS, Lenhart G, Abdalla JA (2006) Patterns of initial pharmacotherapy for Parkinson’s disease in the United States. J Geriatr Psychiatry Neurol 19, 91–97. [DOI] [PubMed] [Google Scholar]
- [20]. Butt DA, Tu K, Young J, Green D, Wang M, Ivers N, Jaakkimainen L, Lam R, Guttman M (2014) A validation study of administrative data algorithms to identify patients with Parkinsonism with prevalence and incidence trends. Neuroepidemiology 43, 28–37. [DOI] [PubMed] [Google Scholar]
- [21]. Fullard ME, Thibault DP, Hill A, Fox J, Bhatti DE, Burack MA, Dahodwala N, Haberfeld E, Kern DS, Klepitskava OS, Urrea-Mendoza E, Myers P, Nutt J, Rafferty MR, Schwalb JM, Shulman LM, Willis AW; Parkinson Study Group Healthcare Outcomes and Disparities Working Group (2017) Utilization of rehabilitation therapy services in Parkinson disease in the United States. Neurology 89, 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40, 373–383. [DOI] [PubMed] [Google Scholar]
- [23]. Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45, 613–619. [DOI] [PubMed] [Google Scholar]
- [24]. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43, 1130–1139. [DOI] [PubMed] [Google Scholar]
- [25]. Espay AJ and Lang AE (2017) Common myths in the use of levodopa in Parkinson disease: When Clinical trials misinform clinical practice. JAMA Neurol 74, 633–634. [DOI] [PubMed] [Google Scholar]
- [26]. Peretz C, Segev H, Rozani V, Gurevich T, El-Ad B, Tsamir J, Giladi N (2016) Comparison of selegiline and rasagiline therapies in Parkinson disease: A real-life study. Clin Neuropharmacol 39, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Weiner WJ (2004) Initial treatment of Parkinson disease: Levodopa or dopamine agonists. Arch Neurol 61, 1966–1969. [DOI] [PubMed] [Google Scholar]
- [28]. DeMaagd G, Philip A (2015) Parkinson’s disease and its management. Pharm Ther 40, 504–532. [PMC free article] [PubMed] [Google Scholar]
- [29]. PD MED Collaborative Group (2014) Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): A large, open-label, pragmatic randomised trial. Lancet 384, 1196–1205. [DOI] [PubMed] [Google Scholar]
- [30]. Searles Nielsen S, Warden MN, Camacho-Soto A, Willis AW, Wright BA, Racette BA (2017) A predictive model to identify Parkinson disease from administrative claims data. Neurology 89, 1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Schrag A, Horsfall L, Walters K, Noyce A, Petersen I (2015) Prediagnostic presentations of Parkinson’s disease in primary care: A case-control study. Lancet Neurol 14, 57–64. [DOI] [PubMed] [Google Scholar]
- [32]. Bertoni JM, Sprenkle PM, Strickland D, Noedel N (2006) Evaluation of Parkinson’s disease in entrants on the Nebraska State Parkinson’s Disease Registry. Mov Disord 21, 1623–1626. [DOI] [PubMed] [Google Scholar]
- [33]. Ishihara LS, Cheesbrough A, Brayne C, Schrag A (2007) Estimated life expectancy of Parkinson’s patients compared with the UK population. J Neurol Neurosurg Psychiatry 78, 1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T (2016) The incidence of Parkinson’s disease: A Systematic review and meta-analysis. Neuroepidemiology 46, 292–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.