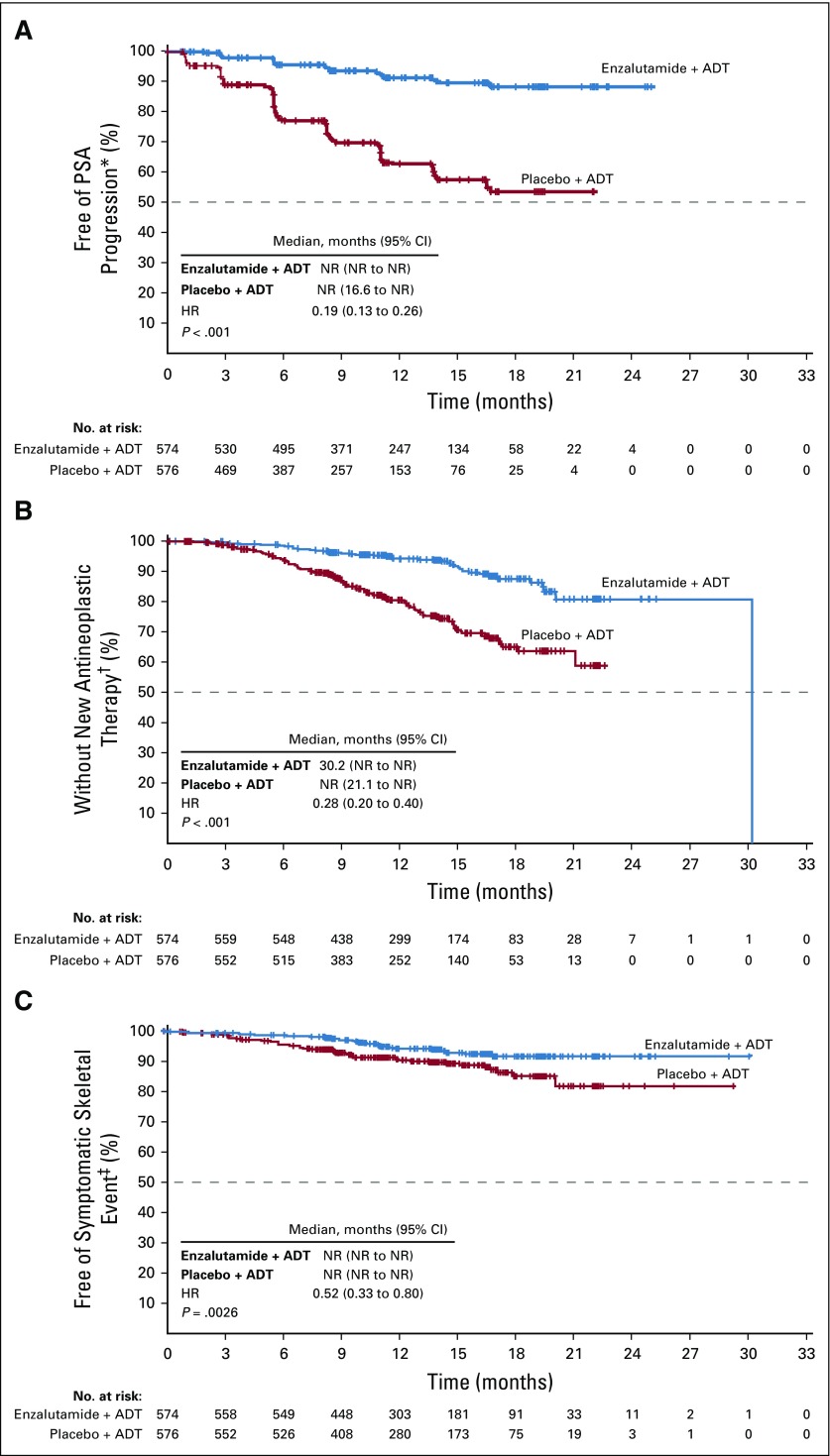
FIG 3.
Kaplan-Meier estimates of time to (A) prostate-specific antigen (PSA) progression, (B) initiation of new antineoplastic therapy, and (C) first symptomatic skeletal event (intent-to-treat population). The dashed line at the 50th percentile indicates the median. Crosses indicate censored data. (*) In patients with no PSA progression, time to PSA progression was censored on the date of the last PSA sample taken. Patients without PSA progression before two or more consecutive missed PSA assessments were censored on the date of last PSA assessment before the assessments missed. (†) In patients with no new antineoplastic therapy initiated for prostate cancer after randomization, time to start of new antineoplastic therapy was censored on the last visit date or the date of randomization, whichever occurred last. The median for the enzalutamide plus androgen deprivation therapy (ADT) group was not a reliable estimate because it resulted from an event observed in the only remaining patient at risk at approximately 30 months, leading to the vertical drop at the end of the Kaplan-Meier curve. The hazard ratio (HR; 95% CI) is a more accurate depiction of the differences between treatment arms. (‡) In patients with no symptomatic skeletal event by the time of the data cutoff point, time to symptomatic skeletal event was censored on the last visit date or the date of randomization, whichever occurred last. HR, hazard ratio; NR, not reached.