Abstract
Cystic fibrosis (CF) is caused by mutations of the gene encoding the CF transmembrane conductance regulator. It remains unclear whether the abnormal immune response in CF involves extrinsic signals released from the external or internal environment. We sought to characterize the peripheral immune signatures in CF and its association with clinical phenotypes. Healthy peripheral blood mononuclear cells (PBMCs) were cultured with plasma from CF probands (CFPs) or healthy control subjects (HCs) followed by nCounter gene and microRNA (miRNA) profiling. A discovery cohort of 12 CFPs and 12 HCs and a validation cohort of 103 CFPs and 31 HCs (our previous microarray data [GSE71799]) were analyzed to characterize the composition of cultured immune cells and establish a miRNA‒mRNA network. Cell compositions and miRNA profiles were associated with clinical characteristics of the cohorts. Significantly differentially expressed genes and abundance of myeloid cells were downregulated in PMBCs after culture with CF plasma (P < 0.05). Top-ranked miRNAs that increased in response to CF plasma (adjusted P < 0.05) included miR-155 and miR-146a, which target many immune-related genes, such as IL-8. Pseudomonas aeruginosa infection was negatively associated with abundance of monocytes and the presence of those regulatory miRNAs. Extrinsic signals in plasma from patients with CF led to monocyte inactivation and miRNA upregulation in PBMCs. An improved understanding of the immune effects of extrinsic factors in CF holds great promise for integrating immunomodulatory cell therapies into current treatment strategies in CF.
Keywords: cystic fibrosis, gene expression, peripheral blood mononuclear cells, immune response, microRNA
Clinical Relevance
A novel transcriptomic profiling–based immune cell analysis was developed to study the extrinsic regulation of immune cells in peripheral circulation of patients with cystic fibrosis (CF). Our study suggests a blunted immune response may exist in patients with CF and supports development of cell immunomodulation therapy in CF.
Cystic fibrosis (CF) is a life-limiting disease caused by mutation of the CF transmembrane conductance regulator (CFTR) gene, which encodes a chloride channel that regulates mucociliary clearance in the airways. Functional failure of CFTR disrupts the airway host defense and results in chronic lung infection, the major cause of the morbidity and mortality in CF (1). The CF airway produces excess proinflammatory cytokines, whereas airway immune cells are deficient in recognizing and clearing pathogens (2). The opportunistic pathogen, Pseudomonas aeruginosa, causes the most prevalent chronic infection found in CF, and often leads to respiratory exacerbations and precipitous declines in lung function (3). The molecular etiology of the impaired immune response in CF remains controversial (4).
Immune cells are central to the infectious and pulmonary pathology of CF (5). Gene expression profiles in blood cells have been investigated to identify predictive markers of the immune response (6, 7). Airway epithelial dysfunction and host–pathogen interactions in patients with CF lead to the release of inflammatory mediators that disrupt the recruitment of leukocytes via the blood circulation (8). Monocyte/macrophage-released IFN, such as IL-6, IL-8, and IL-1α/1β, are involved in the pathogenesis of CF (8), and IL-6 and IL-8 correlate with clinical phenotypes, such as P. aeruginosa infection status (9, 10). microRNAs (miRNAs) are increasingly recognized as potential disease biomarkers and novel targets for various therapies (11). Altered miRNA‒mRNA associations (such as miR-155/IL-8) have been studied in CF epithelial cells and shown to be associated with CF lung function (12).
New CFTR modulator treatments have effectively restored CFTR function in a subgroup of patients, but little is known regarding improvement in the innate immune response (13). The immune response in CF has been extensively studied in CFTR-defective cells, whereas the impacts of extrinsic signals in CFTR wild-type or CFTR-corrected cells remain poorly defined. To address this issue, we and others have studied normal (CFTR wild-type) peripheral blood mononuclear cells (PBMCs) to identify CF plasma–induced changes in transcriptional signatures (14). As all inflammatory mediators and immune cells reach the CF lung via the bloodstream, PBMC is a relevant model in which to study the extrinsic regulation of immune response in CF (15).
Here, we characterized the molecular- and cellular-level responses of normal PBMCs to CF plasma and the association of these signatures to clinical characteristics of CF. Our study revealed that extrinsic factors modify the innate immune response in CF, and support further research into the therapeutic potential of immunomodulation strategies for CF.
Methods
Detailed methods are presented in the data supplement.
Study Subjects and Workflow
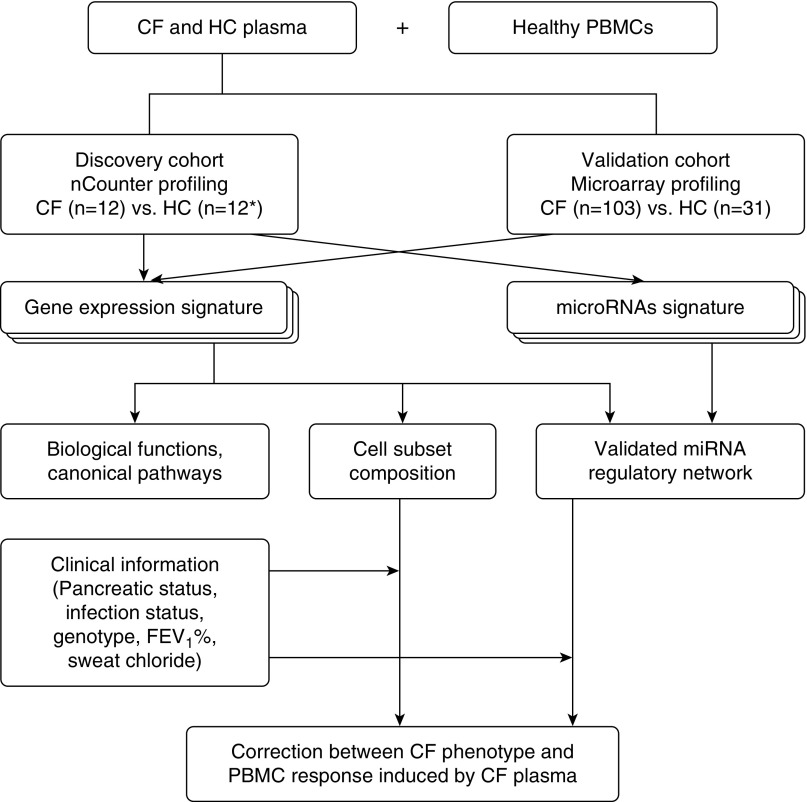
Study subjects were recruited from the Children’s Hospital of Wisconsin and Ann and Robert H. Lurie Children’s Hospital of Chicago as approved by the Institutional Review Boards (CHW 07/72, GC 390, CTSI 847, CHW 01-15, 2015-400). Informed consent was obtained from subjects or their parents/legal guardians. Positive P. aeruginosa infection was reported as one positive microbiological growth from specimens within six consecutive months of study enrollment. A discovery cohort of 12 randomly selected CF probands (CFPs) and 12 unrelated healthy controls (HCs) was recruited (Figure 1). The analyses were validated in a second cohort (validation cohort) of 103 CFPs and 31 HCs, as previously profiled using the Human Genome U133 Plus 2.0 Array (GSE71799) (16) and an additional cohort of 6 CFPs. Plasma or serum samples were collected for use in a PBMC stimulation assay.
Figure 1.
Workflow diagram. Cross-platform assessment of gene expression and microRNA (miRNA) profiles, cellular and molecular signature analysis, and their clinical correlations in plasma-stimulated peripheral blood mononuclear cells (PBMCs). *Healthy control (HC) sample, n = 12 in gene expression analysis and n = 6 in miRNA analysis. CF = cystic fibrosis; FEV1 = forced expiratory volume in 1 second.
PBMC Stimulation and RNA Processing
Plasma was collected from each CF and HC in the discovery cohort. Healthy human PBMCs (UPN727; Cellular Technology Limited) (14, 17) were cocultured with either 20% or 40% of autologous plasma or plasma from the enrolled subjects for 9 hours, and then total RNA was isolated using Trizol (Invitrogen) (14) or the Direct-zol RNA kit (Zymo Research).
Expression Profiling of Immune-related Genes and miRNAs
At least 100 ng total RNA per sample was submitted for nCounter profiling (NanoString Technologies). Immune-related genes and miRNAs were profiled using the Human Immunology v2 Panel and Human v3 miRNA Assay, respectively. Data were normalized and processed using bioinformatics software (nSolver 3.0; NanoString).
Immune Cell Subset Composition Analysis
Cell composition analysis for the discovery cohort was performed using the Immune Cell Type Profiling Module in nSolver 3.0 (NanoString), and cell composition analysis for the validation cohort was performed using a novel signature matrix optimized for human PBMC deconvolution (details in the data supplement).
CD14+ CD16− Monocytes Isolation and Analysis
Healthy human PBMCs (UPN727; Cellular Technology Limited) were seeded (7.5 × 105 per well) and cultured with serum for 9 hours. The serum samples were collected from 6 HCs and 24 CFPs randomly selected from the validation cohort. The surviving PBMCs were counted (n of PBMCs) and CD14+ CD16− monocytes were isolated using the EasySep Human Monocyte Isolation Kit (Stemcell) according to the manufacturer’s instructions (details in the data supplement).
Bioinformatics and Statistical Analysis
Bioinformatics analyses were performed through nSolver 3.0 (NanoString), ingenuity pathway analysis (IPA), and databases (miRTarBase and miRNet) (18, 19). Demographic and clinical characteristics were summarized as median (range) or n (%), and were compared by Mann-Whitney-Wilcoxon, Kruskal-Wallis, or Wilcoxon matched-pairs tests. Associations were examined by chi-square, Fisher’s exact, or Pearson and Spearman correlation tests. Statistical analyses were performed and illustrated using SAS 9.4 (SAS Institute), SPSS 24 (SPSS), Prism 7 (GraphPad Software), and Morpheus (Broad Institute). A P value less than 0.05, without multiple testing adjustment, was considered statistically significant.
Results
Characteristics of the Discovery and Validation Cohorts
The demographic and clinical characteristics of the discovery and validation cohorts are summarized in Table 1. The median age of the CFPs was 15 years in both cohorts, and the sex ratio was well balanced. In each cohort, 58.3% and 63.1%, respectively, of the CFPs were CFTR F508 del homozygous, and more than 80% had severe pancreatic insufficiency. The percentages of probands infected with P. aeruginosa within 6 months (P. aeruginosa positive) were 50% (6 of 12) in the discovery cohort and 41.8% (43 of 103) in the validation cohort. A broad range of sweat chloride and forced expiratory volume in 1 second (FEV1)% values were reported in both cohorts. The median FEV1% values in the P. aeruginosa–positive and –negative probands in the discovery cohort were 86.0 and 110.0, respectively, and in the validation cohort were 92.0 and 104.0, respectively. Analysis of all presenting characteristics showed no significant differences between the two cohorts (all P > 0.05).
Table 1.
Demographic and Clinical Profiles of Discovery and Validation Cohorts
| Study Cohort* | Discovery Cohort (Ncounter) | Validation Cohort (Microarray) | P Value |
|---|---|---|---|
| CF probands | n = 12 | n = 103 | |
| Age, yr† | 15.5 (2.0–43.4) | 14.2 (2.0–53.4) | 0.924 |
| Sex | 0.226 | ||
| M | 3 (25.0) | 47 (45.6) | |
| F | 9 (75.0) | 56 (54.4) | |
| Genotype | 0.180 | ||
| F508del homozygous | 7 (58.3) | 63 (61.2) | |
| F508del heterozygous | 3 (25.0) | 36 (35.0) | |
| F508del absent | 2 (16.7) | 4 (3.9) | |
| Pancreatic status | >0.999 | ||
| Pancreatic insufficiency | 10 (83.3) | 87 (84.5) | |
| Pancreatic sufficiency | 2 (16.7) | 16 (15.5) | |
| Pseudomonas aeruginosa infection within 6 mo | 0.584 | ||
| Yes | 6 (50.0) | 43 (41.8) | |
| No | 6 (50.0) | 60 (58.3) | |
| Sweat chloride, mEq/L | 106.0 (35.4–142.0) (n = 11) | 106.0 (27.7–142.0) (n = 96) | 0.655 |
| FEV1%‡ | 88.0 (56.0–127.0) (n = 11) | 102.0 (46.0–136.0) (n = 94) | 0.451 |
| FEV1% of P. aeruginosa–positive probands‡ | 86.0 (56.0–104.0) (n = 6) | 92.0 (46.0–136.0) (n = 41) | 0.426 |
| FEV1% of P. aeruginosa–negative probands‡ | 110.0 (64.0–127.0) (n = 5) | 104.0 (63.0–128.0) (n = 53) | 0.647 |
| Healthy controls | n = 12 | n = 31 | |
| Age, yr† | 20.5 (6.3–33.2) | 16.5 (6.3–38.5) | 0.705 |
| Sex | 0.775 | ||
| M | 6 (50.0) | 14 (45.2) | |
| F | 6 (50.0) | 17 (54.8) |
Definition of abbreviations: CF = cystic fibrosis; FEV1 = forced expiratory volume in 1 second
Data reported as n (%) or median (minimum-maximum).
Age at sample collection.
Median FEV1% predicted.
Immune-related Gene Expression Profiling
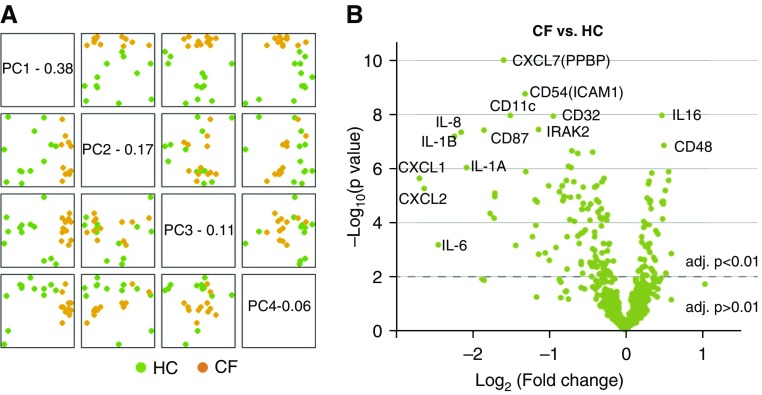
To characterize the abnormal immune response in PBMCs (UPN727 cells), we first profiled the expression of immune-related genes in the nCounter immunology panel in the discovery cohort. Unsupervised hierarchical clustering analysis showed a clear separation of PBMC samples, corresponding to the CF and HC sample groups (Figure E1 in the data supplement). Principal component analysis demonstrated a clear discrimination between CF and HC samples along the top four principal components (Figure 2A). Differentially expressed genes (DEGs) were presented in a volcano plot (Figure 2B). Among all 579 measured genes (probes), a total of 62 DEGs were identified with an adjusted P less than 0.05 and log2 (fold change) greater than 0.5 (Table E1). Of these DEGs, 56 genes (90.3% of total) were downregulated in CF versus HC, including several known CF-associated transcripts, such as those encoding immune receptors (CD11c, CD32, CD87, CD54), cytokine receptors (IL-1A/B, IL-6, IL-8), chemokines (CXCL1, CXCL2, CXCL7), and inflammatory mediators (IRAK [IL-1 receptor–associated kinase-like] 2) (Figure 2B). The DEG results were also summarized at the gene-set level (Table E2) and grouped in genome‐based pathways (Table E3).
Figure 2.
Profiling the differential expression of immune-related genes in CF versus HC samples. (A) Principal component (PC) analysis mapping of study subjects onto informative dimensions. The top four PCs (PC1–4) are plotted against each other for CF versus HC. (B) Volcano plot visualizing the top differentially expressed genes (DEGs), with the log10 (P value; y-axis) plotted against log2 (fold change; x-axis). Highly statistically significant DEGs are denoted in green and the 40 most significant probes are labeled. A few DEGs previously found to be elevated in CF blood or airway are indicated. adj. = adjusted; PPBP = pro-platelet basic protein.
Gene Expression Validation and IPA
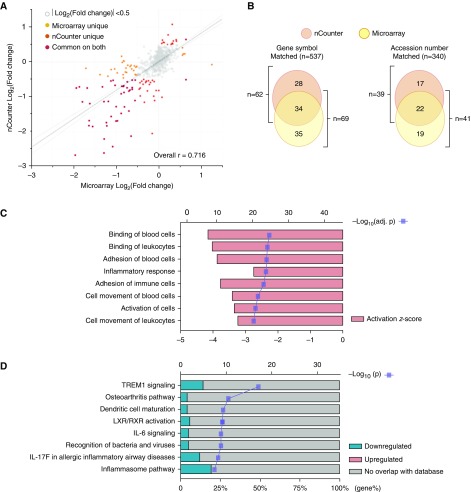
The nCounter gene expression data from the discovery cohort showed a concordance with microarray data from the validation cohort (Pearson correlation coefficient = 0.716, P < 0.001; Figure 3A). Of the 537 genes matched by gene symbol, 62 DEGs (CF vs. HC) were identified from nCounter data (adjusted P < 0.01 and log2 [fold change]| > 0.5), with 34 DEGs shared with the DEGs identified from the microarray data (Figure 3B, left; Table E4). As linked by unique NM_accession number, the total number of matched genes dropped to 340 and the number of shared genes dropped to 22 (Figure 3B, right). The 34 shared DEGs were submitted to the IPA tool to investigate their key biological functions and underlying molecular pathways. CF plasma inhibited (all meaningful z-scores were negative for) the expression of genes involved in many cellular functions of PBMCs, such as cell binding, cell adhesion, and the inflammatory response (Figure 3C and Table E5). Many key signaling pathways were found to be impaired, including the TREM1, IL-6, and IL-17F pathways, and pathways involved in recognition of bacteria and viruses (Figure 3D and Table E6).
Figure 3.
Validation of gene expression signatures between two cohorts/platforms. (A) Scatterplot showing correlation of log2-transformed fold change (CF vs. HC) data between the discovery cohort/nCounter platform and validation cohort/microarray platform. Dots are colored according to the event categories as indicated. The overall Pearson correlation coefficient (r) is given. (B) Number of unique and common DEGs found in the two cohorts/platforms. (C and D) Integrated ingenuity pathway analysis showing the top eight significant biological functions (C) and canonical pathways (D). Log10-transformed P value, activation z-score, and ratio of affected genes are given. LXR/RXR = liver X receptor/retinoid X receptor.
Immune Cell Subset Composition Analysis
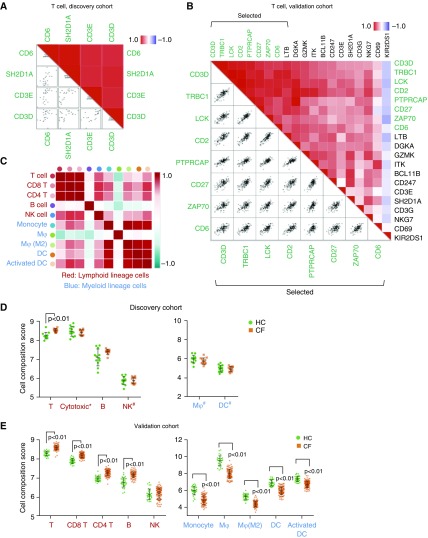
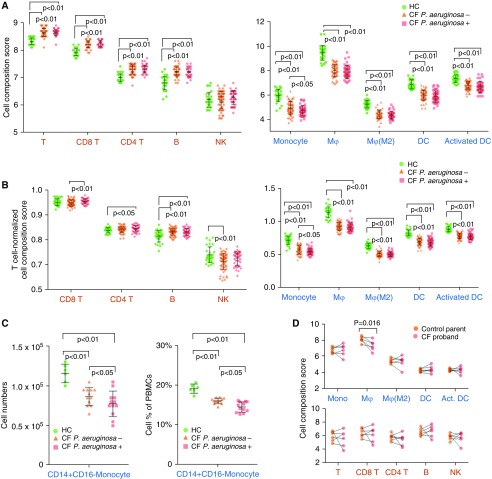
Traditional methods of immune cell profiling, such as flow cytometry, rely on a limited repertoire of phenotypic markers and are constrained by sample and workflow requirements. To gain a better understanding of the shifts in immune cell subsets induced by CF plasma, we enumerated the immune cell subsets in our PBMC samples (UPN727 cells) using a gene-based computational approach (see methods). First, analysis of cell subset composition from the discovery cohort data was performed using predefined marker genes in the nCounter pipeline. Scatter plots were generated to examine the assumption that the marker genes are expressed specifically and uniformly in each cell subset (see detailed methods in the data supplement). Of all 15 cell types tested, 4 cell subsets, including T cells, cytotoxic cells, and B cells, showed strong linear coexpression of each pair of marker genes (P ≤ 0.01; Figures 4A and E2). The immune cell composition analysis demonstrated that the abundance of T cells was significantly higher (P < 0.01) in CF compared with HC; B cells and cytotoxic cells also had greater abundance, but the difference was insignificant (Figure 4D).
Figure 4.
Analysis of immune cell subset composition based on profiling data. (A and B) Pairwise similarity (upper right) and scatter plots matrix (lower left) of predefined marker genes of T cells in the discovery cohort and selected marker genes of T cells in the validation cohort. Gene expression across all subjects in each cohort is shown. (C) All-versus-all heat map of correlation coefficients (Spearman) comparing the composition scores of each cell subset in the validation cohort. (D and E) Composition scores of analyzed cell subsets in the discovery cohort (D) and the validation cohort (E). *Mostly CD8 T cells. #Cells with limited marker genes. B = B cells; DC = dendritic cells; Mφ = macrophages; NK = natural killer cells; T = T cells.
Next, we sought to confirm this result in the validation cohort by using a novel marker gene matrix selected from a previously reported gene matrix (20) (see detailed methods and Table E7). Examples of pairwise similarities in marker genes are shown in Figure 4B (T cells) and Figure E3 (monocytes). Together, we were able to systematically infer the composition of 10 PBMC-derived immune cell subsets, comprised of 5 lymphoid lineage cells (total T cells, CD8 T cells, CD4 T cells, B cells, and natural killer cells) and 5 myeloid lineage cells (monocytes, general and M2 macrophages, and general and activated dendritic cells) (Figure 4C and Table E8). Interestingly, cell compositions of four myeloid cell subsets were significantly higher in CF, whereas all five lymphoid cell subsets were significantly lower (Figure 4E). This result was highly consistent with the results from the discovery cohort, including the three cell subsets with limited marker genes that were not significantly correlated (Figure 4D). We also compared cell subset scores after T cell normalization, which was previously proven to be concordant with flow cytometry measurements (21). Statistically significant differences were found in three lymphoid lineage cells and five myeloid lineage cells (Figure E4).
Clinical Correlation of Plasma–induced Immune Cell Composition
To explore the clinical implications of the CF plasma–induced immune response in PBMCs, we compared the cell composition scores between HC and CF P. aeruginosa–positive and –negative subgroups. As expected, cell composition scores of nine immune cell subsets were significantly changed in individual CF subgroups (P. aeruginosa positive and negative) compared with HC samples (Figure 5A). Most of these cell subsets also showed visible differences between P. aeruginosa–positive and –negative samples, but only the monocyte subset showed a significant difference (P < 0.05; Figure 5A). Similarly, T cell–normalized scores of monocytes and B cells were found to be significantly altered between the two CF subgroups (Figure 5B). To confirm this result, CD14+ CD16− monocytes were isolated from PBMCs (UPN727 cells) by an immunomagnetic separation technique and analyzed by cell counting. Consistent with the cell composition analysis, there were significantly more monocytes from HC subgroups than from the CF subgroups (P. aeruginosa positive and negative), representing a higher cell subset population in PBMCs (19% vs. 16% and 14%; both P < 0.01; Figure 5C). Moreover, the number of monocytes in the P. aeruginosa–negative subgroup was slightly higher than in the P. aeruginosa–positive subgroup (P < 0.05). Next, we compared cell composition scores in CF subgroup samples stratified by pancreatic status and CFTR genotype. We also investigated the correlations of cell composition scores with several continuous variables (age, sex, sweat chloride, and FEV1%). No significant differences or correlations were observed (data not shown).
Figure 5.
Analysis of immune cell subset composition in Pseudomonas aeruginosa–positive and –negative samples. (A and B) Raw (A) and T cell–normalized (B) composition scores of all cell subsets in HC and CF subgroups. (C) Cell numbers (left) and cell subset frequencies (right) of CD14+ CD16− monocytes in PBMCs stimulated by CF and HC serum. (D) Composition scores of 10 major cell subsets in 6 CF probands (CFPs) compared with those of their mothers. Lines connecting the dots indicate pairs of children and mothers. Cell subset scores were evaluated across all 143 subjects in the validation cohort.
The genetic profiling of PBMCs revealed that healthy donor PBMCs, as CFTR wild-type cells show a dramatic immune response to CF plasma. To examine the peripheral immune response in patients with CF, we performed a transcriptome-level expression profiling microarray on PBMCs from a group (n = 6) of young CFPs (three [50%] female, median age = 7.5 [range, 6–16] yr, one [16.7%] P. aeruginosa positive, median sweat chloride = 102.7 [range, 89.5–121.5] mEq/L, and median FEV1% 94.5 [range, 74.0–96.0]) and their mothers as controls (n = 6, median age = 43.5 [range, 35–48] yr). Two CFPs had two copies of the F508 del mutation and the other four probands harbored at least one copy of the F508 del mutation and one copy of another CFTR mutation (G542X, c.2184_2185insA, W128X, and G551D). Using the same immune cell composition approach described previously here, we characterized the composition of 10 PBMC-derived immune cell subsets. Compared with the CF proband mothers (who only have one mutant CFTR gene), there was a significantly lower percentage of macrophages (P = 0.016) in these young CFPs (Figure 5D). Other major peripheral immune subgroups were not different between CFPs and mothers.
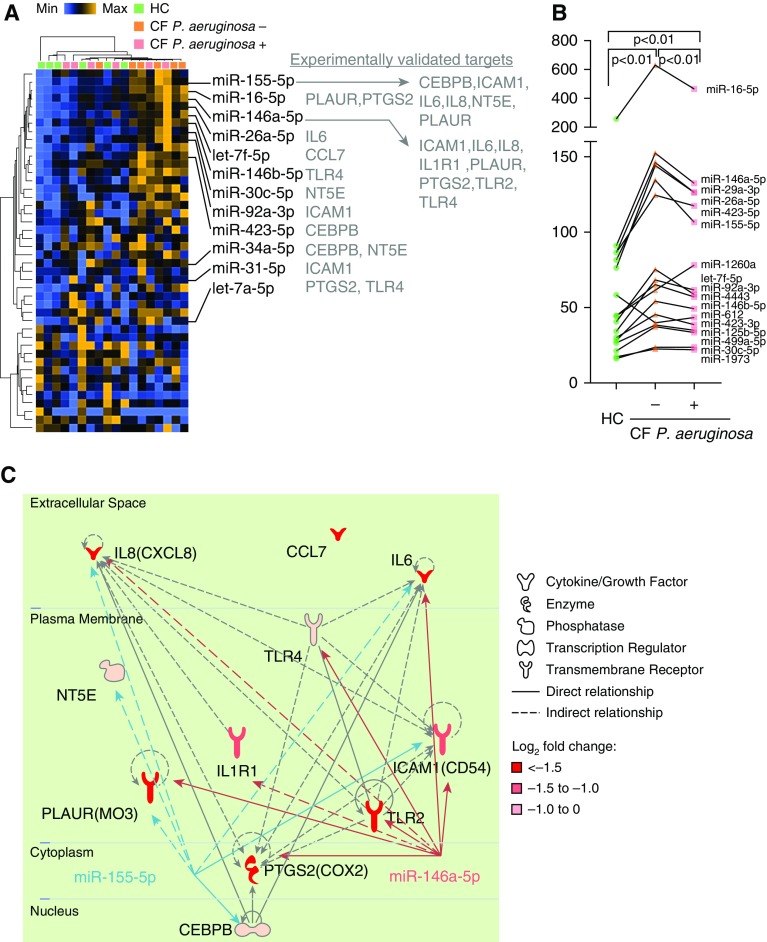
Identification of the miRNA Signature and miRNA Regulatory Network
Considering that the nCounter system generates signals without enzymatic amplification, we hypothesized that significant differences in miRNA expression could potentially be useful for biomarker development. A total of 44 miRNAs were identified as differently expressed between the HC and CF groups (P < 0.05; Figure 6A and Table E9), which also suggested that these miRNAs were differently expressed between CF subgroups (P. aeruginosa positive and negative). Using a commonly accepted method (22), the geometric mean of individual miRNA levels was calculated and compared between combined CF subgroups (P. aeruginosa positive vs. P. aeruginosa negative). Pairwise comparison of 17 combined miRNAs (nCounter signal > 20.0) revealed that these miRNAs could distinguish one subgroup from the other (P < 0.01; Figure 6B). Together, these data suggested that the CF plasma–induced miRNA signature is negatively associated with P. aeruginosa infection.
Figure 6.
Identification of differentially expressed miRNAs and the miRNA regulatory network. (A) Two-way hierarchical clustering of both PBMC samples and significantly differently expressed miRNAs. Orange indicates high expression; blue indicates low expression. (B) nCounter signal levels of top-expressed (signal > 20.0) miRNA signatures in HC and CF P. aeruginosa–positive and CF P. aeruginosa–negative groups. (C) Representative regulatory network including miR-155 and miR-146a, and their mRNA targets and connections. Solid lines indicate direct connections, and dashed lines indicate indirect connections. CEBPB = CCAAT enhancer binding protein β; ICAM-1 = intercellular adhesion molecule 1; NT5E = 5'-nucleotidase ecto; PLAUR(MO3) = plasminogen activator urokinase receptor (monocyte activation antigen Mo3); PTGS2 = prostaglandin-endoperoxide synthase 2; TLR = Toll-like receptor.
Next, the 44 significantly differently expressed miRNAs were integrated with data on the 34 nCounter identified DEGs (n = 34) described previously here. We found and experimentally verified 27 miRNA–target interactions (Figure 6A). Network analysis revealed that 2 miRNA regulators (miR-155 and miR-146a) were hubs that connected 11 out of 12 mRNA targets. As shown in the regulatory network analysis (Figure 6C), at least two direct targets of miR-155 (CEBPB and ICAM) and three of miR-146a (IL-6, Toll-like receptor [TLR] 2/4, and ICAM) were previously confirmed by luciferase reporter assay; the remaining miRNA‒mRNA interactions were validated by indirect evidence.
Discussion
To evaluate the contribution of extrinsic plasma factors to the immune response in CF, we analyzed gene expression and miRNA‒mRNA networks in healthy PBMCs (CFTR wild type, UPN727 cells) in response to CF plasma. We identified the transcriptional regulation of a broad range of immune genes that are targeted by key miRNAs. The gene signatures were associated with immune cell composition scores that were rigorously validated across two independent platforms and cohorts. The miRNA‒mRNA regulatory network was selectively constructed using experimentally verified evidence. Importantly, the immune cell composition and miRNA profiles were associated with P. aeruginosa infection status. In summary, this integrated analysis revealed the impact of P. aeruginosa infection on the innate immune response in CF, independent of the intrinsic CFTR gene defect.
The nCounter analysis system offers advantages over other profiling methods, providing digital output of data and direct measurement without the need for an enzymatic reaction (23). Both nCounter and microarray profiling identified significant downregulation of immune-related genes in PBMCs in response to CF plasma (Figure 3A). These downregulated genes primarily encoded proinflammatory mediators that are released from monocytes and macrophages (Figure 2B). Given that these factors are often increased in CFTR-deficient macrophages/monocytes and contribute to airway inflammation (2, 8, 24), we conclude that the CF plasma–induced immune response may not contribute to the excessive lung inflammation. Instead, the secretion of proinflammatory factors in the CF lung is more likely due to the intrinsic CFTR defect, and may require interactions between neutrophils and epithelial cells (25).
Although CF lung–resident macrophages are hyperinflammatory, a growing amount of evidence suggests that monocytes/macrophages are impaired (26–28). Our IPA showed that many critical cell functions and signaling pathways, such as IL-8, LPS/IL-1, and TREM1 were impaired in response to CF plasma (Figures 3C and 3D). A similar result was previously observed in blood peripheral monocytes isolated from patients with CF (29). Although the intrinsic CFTR defect in alveolar macrophages is known to cause airway inflammation, our study suggests that extrinsic factors acting on immune cells may lead to the blunting of the innate immune response. This conclusion is consistent with reports that circulating CF monocytes release less IL-8 and are hyporesponsive to LPS (30, 31).
Gene-based immune cell profiling provides a clinically practical way to measure the full diversity of immune cells in settings and sample types where traditional methods, such as flow cytometry, are unworkable (32, 33). Computational algorithms are increasingly offering powerful new approaches to enumerate immune cell subsets in tumor tissues, but these methods are less frequently used in PBMC studies (34, 35). Due to the small cohort size and the limited number of marker genes, our analysis of the discovery cohort only evaluated cell composition for four immune cell types (Figures 4A and Figure E2). Given that the marker gene algorithm provided by nSolver 3.0 software (NanoString) was designed for tumor tissue studies, and so far the method to calculate the cell similarity index has only been reported by one group (21), the approach might need to be adjusted and improved for the study of PBMCs. We rigorously evaluated a novel marker gene matrix derived from a previous report (20) from a larger validation cohort (Table E7). This marker gene matrix is PBMC specific, and allowed us to infer the cell composition changes for 10 immune cell subsets (Figure 4C).
Although this new, expanded approach may need to be further validated, we demonstrated the application of technological advances in blood transcriptomics data analysis to distinguish immune cell subsets and assess the pathogen–host interaction in human PBMCs. Importantly, cell composition analysis suggests that lymphoid cells were mostly increased in response to CF plasma, whereas myeloid cells were decreased (Figures 4D and 4E). The decrease in macrophages was also observed in our analysis of PBMCs from a young cohort of CFPs compared with their mothers (Figure 5D). Whereas a broad immune response involving multiple cell subsets was seen in CF plasma–cultured wild-type PBMCs, a similar response was not observed in CF PBMCs; this is likely due to the length of culture, as shorter cell culture (9 h) triggers early cellular actions that would not be detectable in the CF circulating immune cells. These observations are consistent with the well-established concept that CF monocytes/macrophages are unresponsive or deficient in their ability to destroy pathogens (4). Moreover, these data are supported by our prior findings that cell counts of lymphocyte lineage cells (T cells, B cells, and natural killer cells) from CFPs between 6 and 18 years of age mostly remain unchanged compared with HCs (16).
Moreover, using both the gene expression–based computational approach and the traditional cell sorting method, we found a significant shift in monocytes that correlated with P. aeruginosa infection (Figure 5). A previous analysis showed that soluble endotoxin in blood impairs the immune response of circulating monocytes (30). Our results are also consistent with previous findings that P. aeruginosa produces virulence factors that lower macrophage recruitment (36) and stimulate T cells (37). Together, these findings support our hypothesis that the response of peripheral immune cells of patients with CF exposed to a variety of bloodborne pathogens involves both an extrinsic regulatory mechanism in addition to the intrinsic CFTR defect.
miRNAs modulate a large family of genes by inhibiting protein translation, and thus are considered as potential biomarkers for respiratory diseases, including CF (38). Several key miRNAs that affect either CFTR or various key proteins in CF were recognized recently (12, 39). By integrating our newly discovered gene expression and miRNA profiles in PBMCs, we identified miR-155 and miR-146a as the top-ranked miRNAs that responded to CF plasma. These miRNAs target a broad range of immune-related genes, as predicted by miRNA filter analysis (Figure 6A). Although the functional roles of miR-155 and miR-146a/b have been extensively investigated in innate immune cells (40–43), little is known about their expression profiles and gene targets in CF. Previous studies found that miR-155 and miR-146a were upregulated in CF lung epithelial cells (44), circulating CF neutrophils (44), and CF serum (45). Our study revealed that they are elevated in CF plasma–stimulated PBMCs as well.
Furthermore, we found miRNAs that were expressed at higher levels in CF P. aeruginosa–negative samples than in CF P. aeruginosa–positive samples (Figure 6B). This result agrees with a previous finding that P. aeruginosa suppresses miR-155 expression in CF lung epithelial cells (46), suggesting that PBMC miRNAs could be used as biomarkers to sensitively evaluate pathogen interaction in immune cells. Notably, the miRNAs elevated in CF (compared with HC) samples were decreased in CF P. aeruginosa–positive (compared with negative) samples. Therefore, there may be diverse regulatory cascades of miRNAs in the contexts of CF development and P. aeruginosa infection.
It is known that miR-155 and miR-146 regulate proinflammatory genes and contribute to inflammation-mediated injury (41, 47). As discussed previously here, extrinsic factors in CF plasma are more likely to be involved in immune impairment rather than hyperinflammation; thus, we expected to build a miRNA‒mRNA network as identified from PBMCs. Indeed, we identified six miRNA‒mRNA interactions that were previously validated (miR-155: CEBPB/ICAM, miR-146a: IL-6/TLR2/TLR4/PLAUR), and five potential interactions supported by experimental evidence (Figure 6C). Our results also predict that IL-8 might be a direct target of miR-155 in normal PBMCs, although concomitant high expression of miR-155 and IL-8 were found in CF lung endothelial cells (44). Together, our miRNA analysis suggests that CF extrinsic factors induce a cell-context or CFTR genotype-dependent miRNA‒mRNA network.
In summary, we demonstrated that extrinsic factors from CF plasma cause a blunted innate immune response in CFTR wild-type PBMCs. Our study provides novel details and insights into how the internal CF environment affects immune cells, likely including CFTR-corrected immune cells. A clear limitation of this study is that the results and conclusions from this study were made based on single-donor PBMCs (UPN727). Although this cell source has been used as a reporting model to investigate immune response in many diseases (14, 16, 17, 48–51), further validation in primary CF PBMCs will be necessary to draw an unbiased conclusion. Given that cell‒cell interactions were not well characterized in this model, another limitation of this study is that it does not describe the complex interplay between resident and infiltrating immune cells in the lung. Further research is needed to carefully analyze CF PBMCs to evaluate the in vivo immune response of patients developing CF or receiving CFTR modulator therapies. Ultimately, an improved understanding of the complex immune response may inform the integration of immunomodulatory therapies in CF.
Supplementary Material
Acknowledgments
Acknowledgment
The authors acknowledge the assistance of the Cystic Fibrosis (CF) Center staff at Children’s Hospital of Wisconsin in Milwaukee and the Ann & Robert Children’s Hospital of Chicago. They thank all of their patients with CF and their families, and appreciate their involvement and support. They also thank Zainub Ashrafi (Division of Pulmonary Medicine, Department of Pediatrics, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL) for her assistance with data collection.
Footnotes
Supported by the National Heart, Lung, and Blood Institute grant 1DP2OD007031-01 (H.L.), Stanley Manne Children’s Research Institute grant 939001 (H.L.), and Ann and Robert H. Lurie Children’s Hospital (H.L.).
Author Contributions: X.Z. designed and performed the experiments, analyzed and illustrated the data, and wrote the manuscript. A.P. and S.J. performed the statistical analysis. K.W. and K.M. performed subject enrollment. J.E.I., M.J.H., and P.M.S. contributed to experiment design and data analysis. H.L. designed the research, supervised data collection and analysis, and prepared, reviewed, edited, and finalized the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0114OC on March 8, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers. 2015;1:15010. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ralhan A, Laval J, Lelis F, Ballbach M, Grund C, Hector A, et al. Current concepts and controversies in innate immunity of cystic fibrosis lung disease. J Innate Immun. 2016;8:531–540. doi: 10.1159/000446840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marvig RL, Sommer LM, Molin S, Johansen HK. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet. 2015;47:57–64. doi: 10.1038/ng.3148. [DOI] [PubMed] [Google Scholar]
- 4.Ratner D, Mueller C. Immune responses in cystic fibrosis: are they intrinsically defective? Am J Respir Cell Mol Biol. 2012;46:715–722. doi: 10.1165/rcmb.2011-0399RT. [DOI] [PubMed] [Google Scholar]
- 5.Khoury O, Barrios C, Ortega V, Atala A, Murphy SV. Immunomodulatory cell therapy to target cystic fibrosis inflammation. Am J Respir Cell Mol Biol. 2018;58:12–20. doi: 10.1165/rcmb.2017-0160TR. [DOI] [PubMed] [Google Scholar]
- 6.Nick JA, Sanders LA, Ickes B, Briones NJ, Caceres SM, Malcolm KC, et al. Blood mRNA biomarkers for detection of treatment response in acute pulmonary exacerbations of cystic fibrosis. Thorax. 2013;68:929–937. doi: 10.1136/thoraxjnl-2012-202278. [DOI] [PubMed] [Google Scholar]
- 7.Kormann MSD, Dewerth A, Eichner F, Baskaran P, Hector A, Regamey N, et al. Transcriptomic profile of cystic fibrosis patients identifies type I interferon response and ribosomal stalk proteins as potential modifiers of disease severity. PLoS One. 2017;12:e0183526. doi: 10.1371/journal.pone.0183526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, et al. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros. 2012;11:363–382. doi: 10.1016/j.jcf.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 9.De Stefano D, Ungaro F, Giovino C, Polimeno A, Quaglia F, Carnuccio R. Sustained inhibition of IL-6 and IL-8 expression by decoy ODN to NF-κB delivered through respirable large porous particles in LPS-stimulated cystic fibrosis bronchial cells. J Gene Med. 2011;13:200–208. doi: 10.1002/jgm.1546. [DOI] [PubMed] [Google Scholar]
- 10.Carpagnano GE, Barnes PJ, Geddes DM, Hodson ME, Kharitonov SA. Increased leukotriene B4 and interleukin-6 in exhaled breath condensate in cystic fibrosis. Am J Respir Crit Care Med. 2003;167:1109–1112. doi: 10.1164/rccm.200203-179OC. [DOI] [PubMed] [Google Scholar]
- 11.Gunaratne PH, Creighton CJ, Watson M, Tennakoon JB. Large-scale integration of microRNA and gene expression data for identification of enriched microRNA–mRNA associations in biological systems. Methods Mol Biol. 2010;667:297–315. doi: 10.1007/978-1-60761-811-9_20. [DOI] [PubMed] [Google Scholar]
- 12.Sonneville F, Ruffin M, Guillot L, Rousselet N, Le Rouzic P, Corvol H, et al. New insights about miRNAs in cystic fibrosis. Am J Pathol. 2015;185:897–908. doi: 10.1016/j.ajpath.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Brodlie M, Haq IJ, Roberts K, Elborn JS. Targeted therapies to improve CFTR function in cystic fibrosis. Genome Med. 2015;7:101. doi: 10.1186/s13073-015-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy H, Wang X, Kaldunski M, Jia S, Kramer J, Pavletich SJ, et al. Transcriptional signatures as a disease-specific and predictive inflammatory biomarker for type 1 diabetes. Genes Immun. 2012;13:593–604. doi: 10.1038/gene.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saavedra MT, Hughes GJ, Sanders LA, Carr M, Rodman DM, Coldren CD, et al. Circulating RNA transcripts identify therapeutic response in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2008;178:929–938. doi: 10.1164/rccm.200803-387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy H, Jia S, Pan A, Zhang X, Kaldunski M, Nugent ML, et al. Identification of molecular signatures of cystic fibrosis disease status with plasma-based functional genomics. Physiol Genomics. 2019;51:27–41. doi: 10.1152/physiolgenomics.00109.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Jia S, Geoffrey R, Alemzadeh R, Ghosh S, Hessner MJ. Identification of a molecular signature in human type 1 diabetes mellitus using serum and functional genomics. J Immunol. 2008;180:1929–1937. doi: 10.4049/jimmunol.180.3.1929. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y, Siklenka K, Arora SK, Ribeiro P, Kimmins S, Xia J. miRNet—dissecting miRNA–target interactions and functional associations through network-based visual analysis. Nucleic Acids Res. 2016;44:W135–W141. doi: 10.1093/nar/gkw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou CH, Shrestha S, Yang CD, Chang NW, Lin YL, Liao KW, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46:D296–D302. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danaher P, Warren S, Dennis L, D’Amico L, White A, Disis ML, et al. Gene expression markers of tumor infiltrating leukocytes. J Immunother Cancer. 2017;5:18. doi: 10.1186/s40425-017-0215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Severin ME, Lee PW, Liu Y, Selhorst AJ, Gormley MG, Pei W, et al. MicroRNAs targeting TGFβ signalling underlie the regulatory T cell defect in multiple sclerosis. Brain. 2016;139:1747–1761. doi: 10.1093/brain/aww084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhooghe B, Noël S, Huaux F, Leal T. Lung inflammation in cystic fibrosis: pathogenesis and novel therapies. Clin Biochem. 2014;47:539–546. doi: 10.1016/j.clinbiochem.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Paemka L, McCullagh BN, Abou Alaiwa MH, Stoltz DA, Dong Q, Randak CO, et al. Monocyte derived macrophages from CF pigs exhibit increased inflammatory responses at birth. J Cyst Fibros. 2017;16:471–474. doi: 10.1016/j.jcf.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorio C, Montresor A, Bolomini-Vittori M, Caldrer S, Rossi B, Dusi S, et al. Mutations of cystic fibrosis transmembrane conductance regulator rene cause a monocyte-selective adhesion aeficiency. Am J Respir Crit Care Med. 2016;193:1123–1133. doi: 10.1164/rccm.201510-1922OC. [DOI] [PubMed] [Google Scholar]
- 27.Litvack ML, Wigle TJ, Lee J, Wang J, Ackerley C, Grunebaum E, et al. Alveolar-like stem cell–derived Myb(−) macrophages promote recovery and survival in airway disease. Am J Respir Crit Care Med. 2016;193:1219–1229. doi: 10.1164/rccm.201509-1838OC. [DOI] [PubMed] [Google Scholar]
- 28.Lévêque M, Le Trionnaire S, Del Porto P, Martin-Chouly C. The impact of impaired macrophage functions in cystic fibrosis disease progression. J Cyst Fibros. 2017;16:443–453. doi: 10.1016/j.jcf.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 29.del Fresno C, Gómez-Piña V, Lores V, Soares-Schanoski A, Fernández-Ruiz I, Rojo B, et al. Monocytes from cystic fibrosis patients are locked in an LPS tolerance state: down-regulation of TREM-1 as putative underlying mechanism. PLoS One. 2008;3:e2667. doi: 10.1371/journal.pone.0002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.del Campo R, Martínez E, del Fresno C, Alenda R, Gómez-Piña V, Fernández-Ruíz I, et al. Translocated LPS might cause endotoxin tolerance in circulating monocytes of cystic fibrosis patients. PLoS One. 2011;6:e29577. doi: 10.1371/journal.pone.0029577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaman MM, Gelrud A, Junaidi O, Regan MM, Warny M, Shea JC, et al. Interleukin 8 secretion from monocytes of subjects heterozygous for the deltaF508 cystic fibrosis transmembrane conductance regulator gene mutation is altered. Clin Diagn Lab Immunol. 2004;11:819–824. doi: 10.1128/CDLI.11.5.819-824.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons YA, Wu SY, Overwijk WW, Baggerly KA, Sood AK. Immune cell profiling in cancer: molecular approaches to cell-specific identification. NPJ Precision Oncology. 2017;1 doi: 10.1038/s41698-017-0031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altboum Z, Steuerman Y, David E, Barnett-Itzhaki Z, Valadarsky L, Keren-Shaul H, et al. Digital cell quantification identifies global immune cell dynamics during influenza infection. Mol Syst Biol. 2014;10:720. doi: 10.1002/msb.134947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression Genome Biol 201617218[Published erratum appears in Genome Biol 17:249.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z, Huang A, Sun J, Jiang T, Qin FX, Wu A. Inference of immune cell composition on the expression profiles of mouse tissue. Sci Rep. 2017;7:40508. doi: 10.1038/srep40508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cigana C, Lorè NI, Riva C, De Fino I, Spagnuolo L, Sipione B, et al. Tracking the immunopathological response to Pseudomonas aeruginosa during respiratory infections. Sci Rep. 2016;6:21465. doi: 10.1038/srep21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epelman S, Neely GG, Ma LL, Gjomarkaj M, Pace E, Melis M, et al. Distinct fates of monocytes and T cells directly activated by Pseudomonas aeruginosa exoenzyme S. J Leukoc Biol. 2002;71:458–468. [PubMed] [Google Scholar]
- 38.Maltby S, Plank M, Tay HL, Collison A, Foster PS. Targeting microRNA function in respiratory diseases: mini-review. Front Physiol. 2016;7:21. doi: 10.3389/fphys.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKiernan PJ, Greene CM. MicroRNA dysregulation in cystic fibrosis. Mediators Inflamm. 2015;2015:529642. doi: 10.1155/2015/529642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vigorito E, Kohlhaas S, Lu D, Leyland R. miR-155: an ancient regulator of the immune system. Immunol Rev. 2013;253:146–157. doi: 10.1111/imr.12057. [DOI] [PubMed] [Google Scholar]
- 41.Schulte LN, Westermann AJ, Vogel J. Differential activation and functional specialization of miR-146 and miR-155 in innate immune sensing. Nucleic Acids Res. 2013;41:542–553. doi: 10.1093/nar/gks1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Testa U, Pelosi E, Castelli G, Labbaye C. miR-146 and miR-155: two key modulators of immune response and tumor development. Noncoding RNA. 2017;3:E22. doi: 10.3390/ncrna3030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11:163–175. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharyya S, Balakathiresan NS, Dalgard C, Gutti U, Armistead D, Jozwik C, et al. Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J Biol Chem. 2011;286:11604–11615. doi: 10.1074/jbc.M110.198390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montanini L, Smerieri A, Gullì M, Cirillo F, Pisi G, Sartori C, et al. miR-146a, miR-155, miR-370, and miR-708 are CFTR-dependent, predicted FOXO1 regulators and change at onset of CFRDs. J Clin Endocrinol Metab. 2016;101:4955–4963. doi: 10.1210/jc.2016-2431. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchiya M, Kumar P, Bhattacharyya S, Chattoraj S, Srivastava M, Pollard HB, et al. Differential regulation of inflammation by inflammatory mediators in cystic fibrosis lung epithelial cells. J Interferon Cytokine Res. 2013;33:121–129. doi: 10.1089/jir.2012.0074. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, Liu Y, Li L, Su B, Yang L, Fan W, et al. Involvement of inflammation-related miR-155 and miR-146a in diabetic nephropathy: implications for glomerular endothelial injury. BMC Nephrol. 2014;15:142. doi: 10.1186/1471-2369-15-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YG, Cabrera SM, Jia S, Kaldunski ML, Kramer J, Cheong S, et al. Molecular signatures differentiate immune states in type 1 diabetic families. Diabetes. 2014;63:3960–3973. doi: 10.2337/db14-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ideozu JE, Zhang X, Pan A, Ashrafi Z, Woods KJ, Hessner MJ, et al. Increased expression of plasma-induced ABCC1 mRNA in cystic fibrosis. Int J Mol Sci. 2017;18:E1752. doi: 10.3390/ijms18081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palatnik A, Ye S, Kendziorski C, Iden M, Zigman JS, Hessner MJ, et al. Identification of a serum-induced transcriptional signature associated with metastatic cervical cancer. PLoS One. 2017;12:e0181242. doi: 10.1371/journal.pone.0181242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cabrera SM, Wang X, Chen YG, Jia S, Kaldunski ML, Greenbaum CJ, et al. Type 1 Diabetes TrialNet Canakinumab Study Group; AIDA Study Group. Interleukin-1 antagonism moderates the inflammatory state associated with type 1 diabetes during clinical trials conducted at disease onset. Eur J Immunol. 2016;46:1030–1046. doi: 10.1002/eji.201546005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.