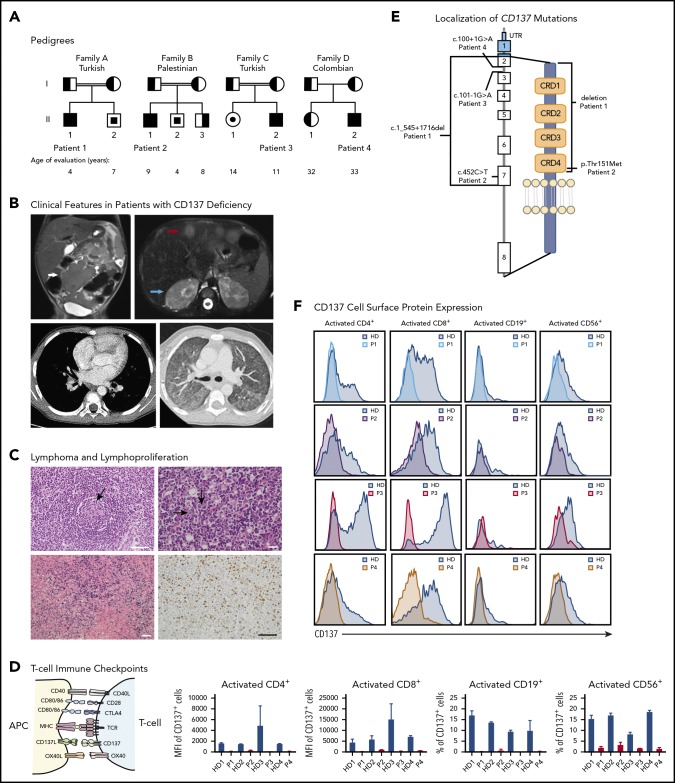
Figure 1.
Genetic and clinical presentation of 4 patients with immune dysregulation. (A) Pedigrees showing 4 families with affected individuals (patients) harboring TNFRSF9 mutations. Solid symbols indicate affected persons who were homozygous for the mutant allele; half-solid symbols, heterozygous persons; center solid symbols, unaffected persons homozygous for the mutant allele; circles, female family members; squares, male family members; double lines, consanguinity. (B) Radiographic features in patients with CD137 deficiency. Top left, Coronal T2-HASTE MR demonstrating a lobulated mass of the small intestine (white arrow) in patient 1 (P1). Top right, axial T2-HASTE STIR MR image of upper abdomen depicting hyperintense multiple metastatic liver nodules (red arrow) and bilateral renal cortical hypointense metastatic nodules (blue arrow) in patient 1. Bottom left, Chest computer tomography (CT) scan indicating right-sided hilar lymph nodes in patient 3 (P3). Bottom right, chest CT scan revealing bilateral ground-glass opacities in patient 2 (P2). (C) Top left, sections of right inguinal lymph node tissue (hematoxylin-and-eosin staining) showing numerous small regressive germinal centers (black arrow) in patient 2 (scale bar, 10 µm). Bottom right, Ki-67 immunostain in patient 2, positive in 20% to 30% of the cells exhibiting a moderate lymphocyte proliferative activity (scale bar, 10 µm). Bottom left, in situ hybridization for EBV-encoded small RNAs (EBER) displaying numerous positive cells in blue in patient 3 (scale bar, 200 µm). Top right, Multinucleated Reed-Sternberg cells (black arrows) typical of Hodgkin lymphoma (scale bar, 20 µm) in a resection of a submandibular lymph node in patient 3. (D) Schematic illustration displaying the interaction of ligands expressed on antigen-presenting cells (APCs) with their respective receptors on activated T cells, including the interaction between CD137L and CD137. (E) Localization of CD137 mutations in our patients. CD137 gene and protein domains with the 4 newly identified mutations are indicated. (F) CD137 protein expressions in activated (CD25+) CD4+ and CD8+ T cells, activated (CD86+) CD19+ B cells, and activated (interleukin 2 [IL-2] stimulated) NK cells (CD56+) in patients and healthy donors (HDs) demonstrating complete loss or markedly reduced expression in patients’ cells, measured by flow cytometry. All error bars indicate plus or minus standard error of mean (SEM). CRD, cysteine-rich domain; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; P4, patient 4; UTR, untranslated region.