Abstract
In this issue of Blood, Cherpokova et al have identified for the first time the important role lipid mediators play in both formation and resolution of clots in deep vein thrombosis (DVT). Their discovery, that Resolvin D4 (RvD4) is a critical specialized proresolving mediator that limits NETosis in the formation of the clot, as well as regulating the rate of clot resolution, identifies a new target for therapeutic intervention and reinforces the important role lipid metabolites play in regulation of blood clot formation under pathologic conditions.1
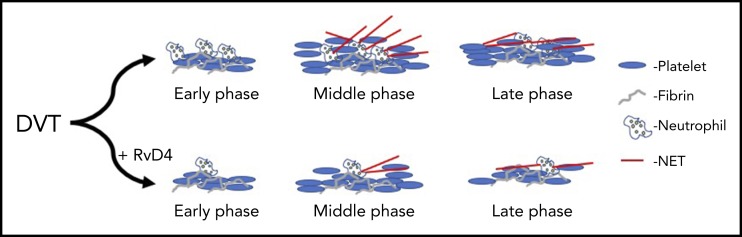
RvD4 attenuates both clot formation and resolution. With treatment of RvD4, DVT clots show fewer neutrophils, reduced NETosis, and faster rate of resolution at late phase after induction of a DVT clot.
Although there is currently no national surveillance of DVT, it is known to be a common thrombotic disorder in the United States, with an annual incidence rate calculated to be at least 1 to 2 people per 1000 people in the population.2 Much work has gone into understanding the underlying genetic and idiopathic risk factors, as well as the temporal biochemical contributors to the development of DVT. However, our understanding of clot resolution in DVT is limited. Because the major risk for mortality in DVT is clot embolization resulting in a pulmonary embolism, termed venous thromboembolism, the primary prevention strategy for venous thromboembolism has focused on prevention of venous clot formation and early recognition of DVT, should it occur. The current prevention strategy, which is predominantly composed of anticoagulation through inhibition of factor Xa or factor IIa, results in inhibition of activation of thrombin, thus reducing the risk for fibrin-rich clot formation and platelet activation in the vessel. Further complicating diagnosis and treatment, DVT formation is known to be facilitated by the innate immune system through neutrophil activation and formation of neutrophil extracellular traps (NETs).3 These NETs trap red blood cells and platelets and stabilize the growing DVT until the clot is finally embolized and travels to the lung. Although our understanding of the formation and growth of the DVT clot is extensive, the mechanisms involved in clot resolution have not been well-characterized.
Much attention has been focused recently on the formation of lipid metabolites in the blood and their prothrombotic or antithrombotic effects in inflammation and clot dynamics.4-6 To this end, the discovery of specialized proresolving mediators (SPMs), with their potential for therapeutic use, is relatively new.7,8 Recent work by Serhan and others has established that inflammation in the vessel wall and lung has the potential to be resolved in part through the formation of specialized SPMs that form acutely at the site of inflammation.9,10 Although these SPMs have been shown to facilitate the resolution of inflammation in tissue beds, this has yet to been shown to be relevant in vessels under pathologic flow and shear conditions such as those found with DVT. Cherpokova et al show here for the first time the formation during growth of the thrombus of various SPMs, including a class of SPMs known as resolvins.1 Using mass spectrometry approaches, they successfully identify the kinetics of the formation of SPMs in the clot. They show that the temporal formation is related to clot formation and resolution. SPMs appear to limit clot size. They go on to establish in a series of eloquent physiological, biochemical, and mass spectrometry-based kinetic experiments that the SPM resolvin D4 (RvD4) is key for determining the rate of clot resolution.
Cherpokova et al go on to show that pretreatment with RvD4 regulates the extent of NETosis formation by limiting neutrophil and macrophage recruitment and activation at the site of injury (see figure). This observation is key, as it is the first time SPMs have been shown to regulate clot formation or resolution in vivo in the vessel. Further, it establishes a new approach for potential therapeutic intervention in DVT and prevention of VTE. An important observation in this study was the identification that the timing for the formation of the various SPMs from their fatty acid precursors varied by SPM and could be grouped as either early- or late-forming SPMs. This study clearly demonstrates that fatty acid substrates are metabolized at different stages of clot formation and resolution, and may play direct or indirect regulatory roles in determining clot size and stability in the vessel. It is important to note that many of these SPMs, and RvD4 in particular, are formed through metabolism of the fish oil DHA and not through metabolism of arachidonic acid. Hence, supplementation with DHA or fish oil may decrease the time from clot formation to resolution and partially explain the cardioprotective effects reported with the use of fish oil.9
Cherpokova et al have, through the study presented in this issue, established the importance of fatty acid metabolites and, in particular, SPMs in the regulation of clot formation and resolution.10 It will be interesting to see how many of these metabolites are involved in regulation of thrombotic clot formation vs clot resolution, and if any of these metabolites can be developed for therapeutic intervention with possibly fewer adverse effects than the agents currently available.
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Cherpokova D, Jouvene CC, Libreros S, et al. . Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood. 2019;134(17):1458-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501. [DOI] [PubMed] [Google Scholar]
- 3.Martinod K, Witsch T, Farley K, Gallant M, Remold-O’Donnell E, Wagner DD. Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J Thromb Haemost. 2016;14(3):551-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adili R, Tourdot BE, Mast K, et al. . First selective 12-LOX inhibitor, ML355, impairs thrombus formation and vessel occlusion in vivo with minimal effects on hemostasis. Arterioscler Thromb Vasc Biol. 2017;37(10):1828-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duchez AC, Boudreau LH, Naika GS, et al. . Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA [published correction appears in Proc Natl Acad Sci U S A. 2015;112(49):E6825]. Proc Natl Acad Sci USA. 2015;112(27):E3564-E3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung J, Hawley M, Holinstat M. The expansive role of oxylipins on platelet biology. J Mol Med (Berl). 2017;95(6):575-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalli J, Serhan CN. Identification and structure elucidation of the pro-resolving mediators provides novel leads for resolution pharmacology. Br J Pharmacol. 2019;176(8):1024-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Aspects Med. 2017;58:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schunck WH, Konkel A, Fischer R, Weylandt KH. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol Ther. 2018;183:177-204. [DOI] [PubMed] [Google Scholar]
- 10.Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016;30(8):2792-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]