Abstract
The etiology of sudden hearing loss (SHL) has not been fully elucidated. Previous studies claimed that different etiological factors may play a role in the pathogenesis of SHL. The aim of the present study is to investigate the presence of oxidative stress (OS) in SHL cases using thiol-disulfide balance. In addition, total oxidant status (TOS), total antioxidant status (TAS), oxidative stress index (OSI) and lipid hydroperoxide levels (LOOH) were investigated. A total of 30 cases (15 female, 15 male, mean age 48.9 ± 8.1 years, age range: 36–68 years) were included in the study. Thiol and disulfide amounts, thiol/disulfide ratios, TOS, TAS, OSI and LOOH scores of the case group and control group were compared. Native thiol (SH) and total thiol (SH + SH) values were significantly lower in the SHL group than in the control group (p = 0.028 and p = 0.044, respectively). The LOOH value, TOS value and OSI value were significantly higher in the SHL group than in the control group (all p values < 0.05). The TAS value was significantly lower in the SHL group than in the control group (p = 0.0001). The present study has presented that the thiol-disulfide balance was impaired in SHL cases. OS may play a role in the development of SHL.
Keywords: Oxidative stress, Sudden hearing loss, Etiology, Thiol-disulfide balance
Introduction
Sudden hearing loss (SHL) is defined as 30-dB or more hearing loss at 3 consecutive frequencies in the last three days according to Clinical Practice Guideline on Sudden Hearing Loss published by American Academy of Otolaryngology in 2012 [1]. SHL is one of the otological emergencies, its prevalence is thought to be around 5–20/100,000 [2, 3]. SHL is usually unilateral, although in less than 5% of cases it is observed as bilateral SHL. Even though it is frequently detected in patients at the age of 50 to 60, it may be observed in young patients who have not had any comorbidities [4].
The etiology has not been fully elucidated. Previous studies claimed that different etiological factors may play a role in the pathogenesis of SHL. In a recent meta-analysis study, it was reported that the possible etiological causes of SHL were infections (12.8%), otological diseases (4.7%), trauma (4.2%), vascular and hematologic diseases (2.8%) and neoplastic diseases (2.3%) [5].
The recent studies on SHL are mostly studies that aim to understand the etiopathogenesis of SHL. Inner ear is more easily affected by vascular pathologies because the labyrinthine artery that feeds the inner ear is an end artery, and doesn’t have collaterals. Endothelial dysfunction causes development of vascular pathologies. The etiologic causes of endothelial dysfunction may play a role in the pathogenesis of SHL [6, 7]. The relationship between oxidative stress (OS) and endothelial dysfunction has been presented in several studies. It has been reported that OS resulting from impairment of oxidant-antioxidant balance in the body may interfere with arterial blood flow of the inner ear by causing endothelial dysfunction [8, 9].
Different methods have been reported for detecting OS in the body. OS in SHL cases has been demonstrated in very few studies [6–8]. In this study, the presence of OS in SHL cases was investigated with thiol-disulfide balance, which is a novel and reliable method. In addition, total oxidant status (TOS), total antioxidant status (TAS), oxidative stress index (OSI) and lipid hydroperoxide levels (LOOH) were investigated and compared with healthy cases.
Methods
This clinical trial was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. The study was approved by the local ethics committee (28/11/2017-E.42520). Informed consent was obtained from all the cases involved in the study.
This non-randomized, prospective study was conducted in the otolaryngology clinic of our hospital. A total of 30 cases (15 female, 15 male, mean age 48.9 ± 8.1 years, age range: 36–68 years) were included in the study. The study included 15 subjects (7 female, 8 male, mean age 48.9 ± 7.5 years, age range: 36–68 years) who met the American Academy of Otolaryngology 2012 Clinical Practice Guideline on Sudden Hearing Loss [1] criteria, as case group. Sudden hearing loss specified as more than 30 dB hearing loss in at least 3 consecutive frequencies in the last 72 h, in accordance with the guidelines of the American Academy of Otolaryngology [1]. The control group consisted of 15 healthy cases (8 females and 7 males, mean age 48.9 ± 8.9 years, age range: 36–62 years) with no sudden hearing loss, no previous otological surgery, no hearing loss history and pure tone audiometry results at normal level were included in the study. Patients with any systemic diseases were excluded from the control groups according to their anamnesis and systemic examination.
Cases with known central and inner ear pathologies, history of previous otological surgery, history of sudden hearing loss, cases with history of malignancy, hematologic, neoplastic and neurological diseases, cases with comorbid diseases (such as diabetes mellitus, hypertension, hypercholesterolemia, chronic renal failure, heart failure), and cases that use anticoagulant and antiaggregant drugs were excluded from the study.
Blood Collection from Cases
Blood samples of cases with sudden hearing loss were collected between 8 a.m. and 10 a.m., after a 12-h fasting before the treatment. Blood samples of control group cases were collected between 8 a.m. and 10 a.m., after a 12-h fasting. Blood samples were centrifuged for 10 min at 4000 rpm, after 30–45 min of waiting, stored at − 80 °C in Eppendorf® tubes until serum is separated.
Measurement of Parameters
Measurement of Thiol/Disulfide
The total thiol content of the blood samples was measured using a modified Ellman’s reagent. The half value of the difference between total thiol and native thiol amounts gave the dynamic disulphide amount. After determining the natural thiol and disulfide levels, the disulfide/thiol ratio was calculated as previously described by Erel and Neselioğlu [10].
Total Antioxidant Status Level
Total antioxidant status levels of the samples (TAS) were measured using Rel Assay brand commercial kits (Rel Assay® Kit Diagnostics, Turkey) with spectrophotometric method (Thermo Scientific Multiskan® GO, ThermoFisher Scientific, Vartaa, Finland). Trolox, a water-soluble analog of vitamin E, was used as the calibrator. The results are expressed in mmol Trolox equiv./lt.
Total Oxidant Status (TOS) Level
Total oxidant status levels of the samples (TAS) were measured using Rel Assay brand commercial kits (Rel Assay® Kit Diagnostics, Turkey) with spectrophotometric method (Thermo Scientific Multiskan® GO, ThermoFisher Scientific, Vartaa, Finland). Hydrogen peroxide was used as the calibrator. The results are expressed in μmol H2O2 equiv./lt.
Calculation of Oxidative Stress Index
While calculating OSI, which is defined as the percentage of the ratio of TOS levels to TAS levels, mmol value in the TAS test was converted to μmol units as in the TOS test. The results were expressed as “arbitrary unit” (AU) and calculated according to the following formula.
Serum Lipid Hydroperoxide Level
Serum lipid hydroperoxide concentrations were measured spectrophotometrically using automatic ferrous oxidation xylenol orange (FOX-2) test.
Thiol and disulfide amounts, thiol/disulfide ratios, TAS, TOS, LOOH and OSI scores of the case group and control group were compared.
Statistical Analysis
Mean, standard deviation, median, lowest, highest, frequency and ratio values were used in the descriptive statistics of the data. Independent Samples t Test, Mann–Whitney U Test was used in the analysis of quantitative independent data. Chi square test was used in the analysis of independent qualitative data. SPSS 22.0 software package was used for analyses.
Results
There was no difference in the age and gender distribution of the cases between the SHL group and the control group (p values > 0.05). Native thiol (SH) and total thiol (SH + SH) values were significantly lower in the SHL group than in the control group (p = 0.028 and p = 0.044, respectively). There was no significant difference in disulphide (SS) values, %SS/SH values, %SS/SH + SS values, %SH/SH + SS values between SHL and control groups (all p values > 0.05).
The LOOH value (Fig. 1), TOS value (Fig. 2) and OSI value (Fig. 3) were significantly higher in the SHL group than in the control group (all p values < 0.05). The TAS value (Fig. 4) was significantly lower in the SHL group than in the control group (p = 0.0001) (Table 1).
Fig. 1.
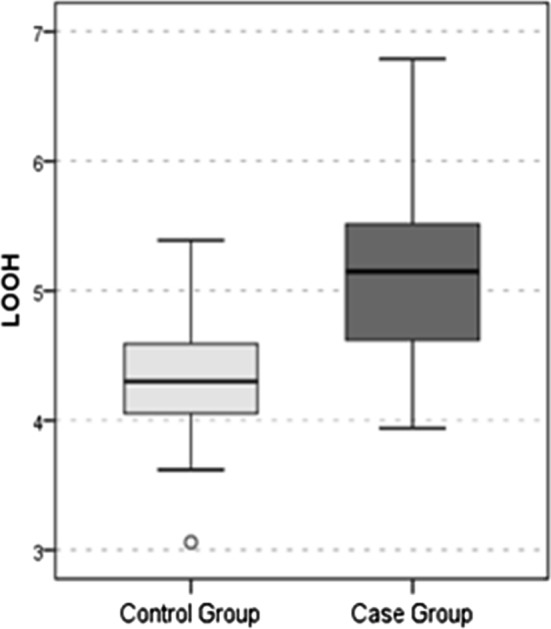
LOOH Value
Fig. 2.
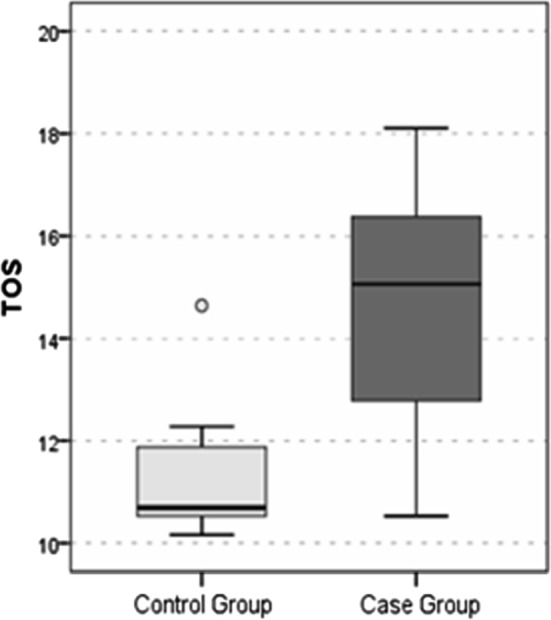
TOS Value
Fig. 3.
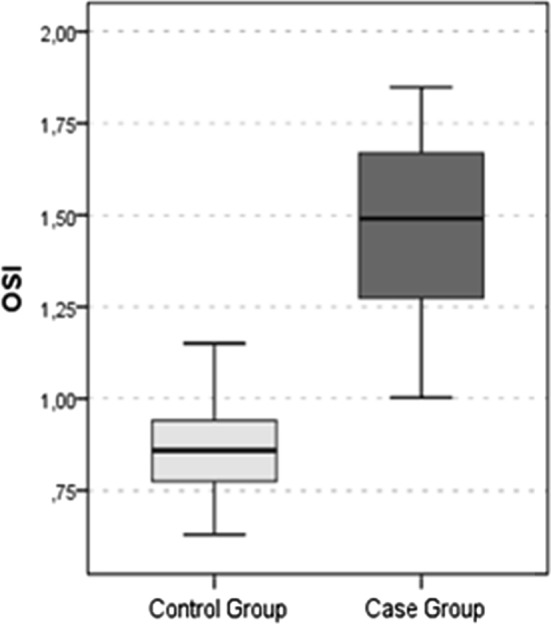
OSI Value
Fig. 4.
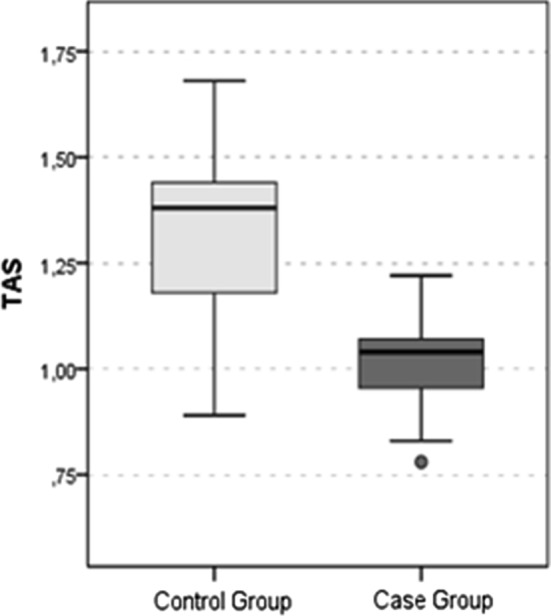
TAS Value
Table 1.
Comparison of biochemical findings between each groups
| Control group (N:15) | Case group (N:15) | p | |||
|---|---|---|---|---|---|
| Mean ± S.D./n (%) | Median | Mean ± S.D./n (%) | Median | ||
| Age | 48.9 ± 7.5 | 48.0 | 48.9± 8.9 | 48.0 | 1.000t |
| Gender | |||||
| Female | 7 (46.7%) | 8 (53.3%) | 0.715X2 | ||
| Male | 8 (53.3%) | 7 (46.7%) | |||
| Native thiol (SH) | 409.6 ± 58.4 | 390.9 | 368.1 ± 37.4 | 365.4 | 0.028t |
| Total thiol (SH + SS) | 442.9 ± 62.8 | 425.6 | 402.5 ± 39.4 | 398.2 | 0.044t |
| Disuplhide (SS) | 16.7 ± 6.3 | 17.4 | 17.2 ± 7.4 | 17.3 | 0.822t |
| % SS/SH | 4.1 ± 1.5 | 4.2 | 4.7 ± 2.1 | 4.7 | 0.346t |
| % SS/SH + SS | 3.8 ± 1.3 | 3.9 | 4.3 ± 1.8 | 4.3 | 0.373t |
| % SH/SH + SS | 92.5 ± 2.6 | 92.2 | 91.5 ± 3.5 | 91.3 | 0.373t |
| LOOH | 4.3 ± 0.6 | 4.3 | 5.2 ± 0.8 | 5.2 | 0.002t |
| TAS | 1.3 ± 0.2 | 1.4 | 1.0 ± 0.1 | 1.0 | 0.0001t |
| TOS | 11.2 ± 1.2 | 10.7 | 14.6 ± 2.3 | 15.1 | 0.0001t |
| OSI | 0.9 ± 0.1 | 0.9 | 1.5 ± 0.3 | 1.5 | 0.0001t |
KMann–whitney u test/X2Chi square test/tStudent’s t test
SD standart deviation, TAS total antioxidan status, TOS total oxidan status, OSİ, oxidative stres index, LOOH lipid hydroperoxide
Discussion
Investigation of the possible etiological factors of SHL, the elimination of these factors and the planning of treatment for these factors in cases with SHL are current research subjects in literature. It is clear that the work to be done in this subject matter will increase our knowledge on the SHL. The discovery of possible etiological factors and the development of appropriate treatment modalities will contribute to the country’s economy as well as preventing the loss of labor force resulting from SHL.
Optimal arterial blood supply and adequate oxygenation are required for full functioning of the inner ear. According to recent studies, healthy and sufficient arterial blood supply depends on the smooth functioning of the endothelial system [11, 12]. As a result of deterioration of endothelial function, disturbance to the blood supply of the inner ear is possible. An increase of the OS in the body results in endothelial damage, which leads to impaired blood flow. Highly oxidant species such as nitric oxide and peroxynitrite lead to increased production of pro-inflammatory cytokines, as well as resulting in endothelial damage. Current literature shows that endothelial dysfunction plays a role in sudden hearing loss [6, 7]. Imbalances in the oxidant-antioxidant system may cause endothelial dysfunction by inducing the production of reactive oxygen species. Berjis et al. [13] reported that they have detected endothelial dysfunction in sudden hearing loss cases. In another study, Capaccio et al. [14] showed that OS molecules increased in cases of sudden hearing loss.
In the human body, there is a balance between oxidant and antioxidant molecules. Certain concentrations of oxidant molecules are required for the maintenance of vital functions in the body. Under normal conditions, the increase in free oxygen radicals in the body stimulate antioxidant systems and the body is protected from the harmful effects of these molecules. But the insufficiency of the antioxidant system and the chronic effects of the oxidant molecules lead to oxidative stress (OS). Studies have shown that the OS leads to many auditory neuropathies [15–17].
A large number of methods have been reported in the literature to show the OS status of the body. However, these methods are mostly clinically impractical for the determination of the OS status as they are relatively costly, hard to measure and take a long time. Thus, simpler and cheaper methods are required [10]. As several diseases result from the prolonged exposure to OS, it is suggested that these diseases are preventable. The elimination and prevention of the cause of the OS may prevent the development of many diseases and improve the quality of life.
Thiols are organic compounds that are made up of hydrogen and sulfur that are bound to a carbon atom. Thiols greatly contribute to the antioxidant system in the body, and play an important role in neutralizing the oxidant molecules. Disulfide bonds bind to these thiol groups and they decrease the amount of available thiol, thus creating the thiol/disulfide balance in the body [18, 19]. The antioxidant molecules play important roles in apoptosis, detoxification, cell renewal, and regulation of the enzymatic activity of the cell [20]. Neselioglu and Erel [10] have developed an automatic and novel method which makes it possible to individually or cumulatively calculate the thiol and disulfide levels. This method has been quickly adopted due to its practicality. There are over one hundred studies that investigate the effects of OS on the pathophysiologies of disorders by using the thiol-disulfide balance [20–24].
When the levels of the thiol group—a vital component in the body—decrease, several systems develop pathological findings. Previous studies indicate that decreased thiol and increased sulfide values are associated with cancer, dermatological disorders, neuropsychological disorders, hematological diseases, chronic inflammatory disorders and rheumatologic diseases [18–26]. Dinç et al. [8] reported that thiol/disulfide metabolism was impaired in cases experiencing sudden hearing loss compared to healthy subjects. The authors reported that thiol count decreased and disulfide level increased in patients with sudden hearing loss [8].
In the present study, the presence of OS in SHL etiology was investigated. The OS parameters were compared between the SHL cases and the control group. It was detected that the thiol levels were lower and the parameters indicating oxidant system (TOS, OSI and LOOH) were higher in the SHL cases compared to the healthy cases. In this study, thiol levels in the SHL cases were found to be lower than the control group, but no significant increase in disulfide levels was observed. The presence of OS in SHL etiology is proved. The presence of low thiol levels suggests that amount of antioxidant molecules decreased in the body. We think that the probable cause of not detecting high disulfide quantities is related to the low number of cases. The strength of this study is that the presence of the OS is not solely assessed by the thiol-disulfide balance, but the presence of the OS is evidenced with parameters such as TOS, TAS, OSI and LOOH. The fact that there are very few studies on this subject in the literature reveals the need to confirm the previous studies with new studies. In this sense, this study is a valuable work. According to the study results, we considered that high levels of OS may be one of the causes or a predisposing factor in SHL. High levels of OS may disrupt the systemic blood circulation, leading to impaired cochlear blood flow and developed SHL. Reducing high levels of OS could reduce the development of systemic diseases and prevent the development of SHL. In addition, measurement of the level of OS to be performed in patients with SHL may be helpful in the regulation of the treatment protocol. The relatively small number of studies in this context hinder full understanding of the pathogenesis of SHL.
The number of cases included in the study remained relatively low, as the study was non-randomized, prospective, and had rigorous inclusion criteria. As a limitation factor, the samples of blood were taken from systemic circulation to evaluate the OS status. However, it is difficult to directly study cochlear microcirculation disturbance or embolism in SSNHL patients due to the deep position of the cochlea within the temporal bone. Another limitation factor is the fact that the OS has not been assessed in the cases with SHL according to treatment response. Thereafter, studies with larger numbers of cases are needed to determine the presence of OS and to investigate the effectiveness of antioxidant treatment methods.
Conclusion
The present study has presented that the thiol-disulfide balance was impaired in SHL cases. OS may play a role in the development of SHL. Thiol-disulfide balance is an effective, inexpensive and reliable method in the detection of OS. The effectiveness of antioxidant treatment for SHL cases should be investigated in future studies.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stachler RJ, Chandrasekhar SS, Archer SM, et al. American academy of otolaryngology-head and neck surgery. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3 suppl):S1–S35. doi: 10.1177/0194599812436449. [DOI] [PubMed] [Google Scholar]
- 2.Alexander TH, Harris JP. Incidence of sudden sensorineural hearing loss. Otol Neurotol. 2013;34:1586–1589. doi: 10.1097/MAO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber BE, Agrup C, Haskard DO, Luxon LM. Sudden sensorineural hearing loss. Lancet. 2010;375:1203–1211. doi: 10.1016/S0140-6736(09)62071-7. [DOI] [PubMed] [Google Scholar]
- 4.Fasano T, Pertinhez TA, Tribi L, Lasagni D, Pilia A, Vecchia L, et al. Laboratory assessment of sudden sensorineural hearing loss: a case-control study. Laryngoscope. 2017;127(10):2375–2381. doi: 10.1002/lary.26514. [DOI] [PubMed] [Google Scholar]
- 5.Chau JK, Lin JR, Atashband S, Irvine RA, Westerberg BD. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope. 2010;120:1011–1021. doi: 10.1002/lary.20784. [DOI] [PubMed] [Google Scholar]
- 6.Gul F, Muderris T, Yalciner G, Sevil E, Bercin S, Ergin M, et al. A comprehensive study of oxidative stress in sudden hearing loss. Eur Arch Otorhinolaryngol. 2017;274(3):1301–1308. doi: 10.1007/s00405-016-4301-1. [DOI] [PubMed] [Google Scholar]
- 7.Quaranta N, De Ceglie V, D’Elia A. Endothelial dysfunction in idiopathic sudden sensorineural hearing loss: a review. Audiol Res. 2016;6(1):151. doi: 10.4081/audiores.2016.151.eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinc ME, Ulusoy S, Is A, Ayan NN, Avincsal MO, Bicer C, et al. Thiol/disulphide homeostasis as a novel indicator of oxidative stress in sudden sensorineural hearing loss. J Laryngol Otol. 2016;130(5):447–452. doi: 10.1017/S002221511600092X. [DOI] [PubMed] [Google Scholar]
- 9.Ballesteros F, Tassies D, Reverter JC, Alobid I, Bernal-Sprekelsen M. Idiopathic sudden sensorineural hearing loss: classic cardiovascular and new genetic risk factors. Audiol Neurootol. 2012;17:400–408. doi: 10.1159/000341989. [DOI] [PubMed] [Google Scholar]
- 10.Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47(18):326–332. doi: 10.1016/j.clinbiochem.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Vassalle C, Pratali L, Boni C, Mercuri A, Ndreu R. An oxidative stress score as a combined measure of the pro-oxidant and antioxidant counterparts in patients with coronary artery disease. Clin Biochem. 2008;41:1162–1167. doi: 10.1016/j.clinbiochem.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Fechter DL. Oxidative stress: a potential basis for potentiation of noise-induced hearing loss. Environ Toxicol Pharmacol. 2005;19:543–546. doi: 10.1016/j.etap.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Berjis N, Moeinimehr M, Hashemi SM, Hashemi SM, Bakhtiari EK, Nasiri S. Endothelial dysfunction in patients with sudden sensorineural hearing loss. Adv Biomed Res. 2016;5:5. doi: 10.4103/2277-9175.174978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capaccio P, Ottaviani F, Cuccarini V, Bottero A, Schindler A, Cesana BM, et al. Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope. 2007;117:547–551. doi: 10.1097/MLG.0b013e31802f3c6a. [DOI] [PubMed] [Google Scholar]
- 15.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 16.Labbé D, Teranishi MA, Hess A, Bloch W, Michel O. Activation of caspase-3 is associated with oxidative stress in the hydropic guinea pig cochlea. Hear Res. 2005;202:21–27. doi: 10.1016/j.heares.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Wangemann P. Free radical stress mediated loss of Kcnj10 protein expression in stria vascularis contributes to deafness in Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007;294:F139–F148. doi: 10.1152/ajprenal.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 2000;72(2 Suppl):653–669. doi: 10.1093/ajcn/72.2.653S. [DOI] [PubMed] [Google Scholar]
- 19.Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem. 2013;288(37):26489–26496. doi: 10.1074/jbc.R113.462929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cvetković T, Vlahović P, Todorović M, Stanković M. Investigation of oxidative stress in patients with chronic tonsillitis. Auris Nasus Larynx. 2009;36:340–344. doi: 10.1016/j.anl.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Eren Y, Dirik E, Neselioglu S, Erel O. Oxidative stress and decreased thiol level in patients with migraine: cross-sectional study. Acta Neurol Belg. 2015;115:643–649. doi: 10.1007/s13760-015-0427-y. [DOI] [PubMed] [Google Scholar]
- 22.Ergin M, Cendek BD, Neselioglu S, Avsar AF, Erel O. Dynamic thiol-disulfide homeostasis in hyperemesis gravidarum. J Perinatol. 2015;35(10):788–792. doi: 10.1038/jp.2015.81. [DOI] [PubMed] [Google Scholar]
- 23.Uysal P, Avcil S, Neşelioğlu S, Biçer C, Çatal F. Association of oxidative stress and dynamic thiol-disulphide homeostasis with atopic dermatitis severity and chronicity in children: a prospective study. Clin Exp Dermatol. 2018;43(2):124–130. doi: 10.1111/ced.13219. [DOI] [PubMed] [Google Scholar]
- 24.Demirseren DD, Cicek C, Alisik M, Demirseren ME, Aktaş A, Erel O. Dynamic thiol/disulphide homeostasis in patients with basal cell carcinoma. Cutan Ocul Toxicol. 2017;36(3):278–282. doi: 10.1080/15569527.2016.1268150. [DOI] [PubMed] [Google Scholar]
- 25.Karadag-Oncel E, Erel O, Ozsurekci Y, Caglayik DY, Kaya A, Gozel MG, et al. Plasma oxidative stress and total thiol levels in crimean-congo hemorrhagic fever. Jpn J Infect Dis. 2014;67(1):22–26. doi: 10.7883/yoken.67.22. [DOI] [PubMed] [Google Scholar]
- 26.McBean GJ, Aslan M, Griffiths HR, Torrão RC. Thiol redox homeostasis in neurodegenerative disease. Redox Biol. 2015;5:186–194. doi: 10.1016/j.redox.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


