Figure 1: Specific interaction between Stg_CT & PSD-95 triggers liquid-liquid phase separation.
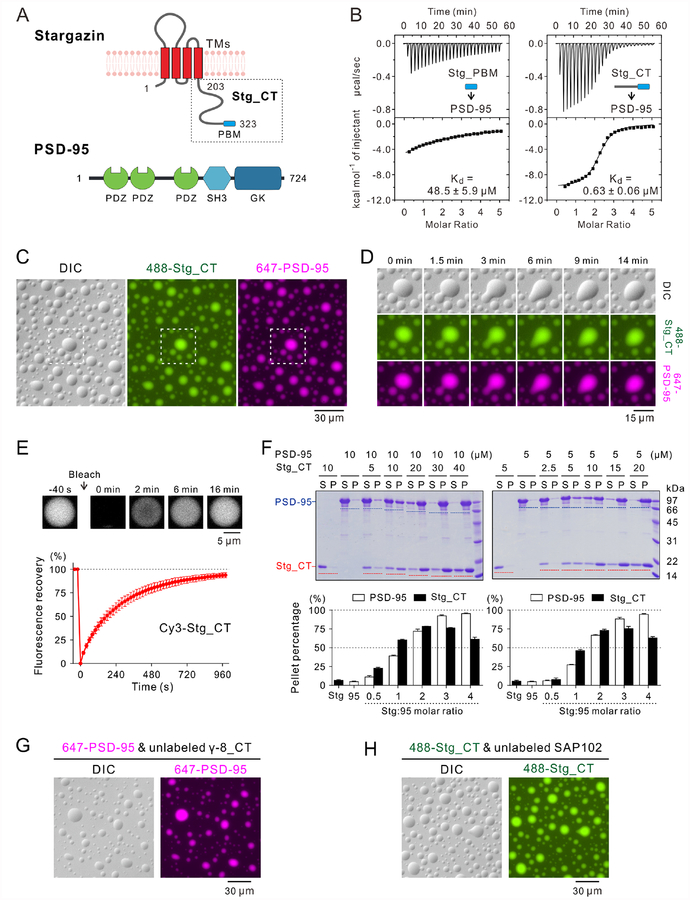
A: Schematic diagram showing the domain organization of Stg and PSD-95.
B: ITC-based measurements comparing PSD-95 binding to Stg_PBM and Stg_CT. Stg_PBM or Stg_CT (250 μM) was titrated into PSD-95 (10 μM).
C: DIC and fluorescence images showing that the mixtures of 30 μM Alexa 488-labeled Stg_CT and 10 μM Alexa 647-labeled PSD-95 formed LLPS at room temperature and both components were highly co-enriched in micron-sized droplets. Only 1% of each protein was labeled by the indicated fluorophores and this labeling ratio was used throughout this study unless otherwise specified. The dashed box is selected for zoom-in analysis in panel D.
D: Zoom-in and time-lapse images showing small droplets coalesced into larger ones (0–9 min) and the morphology of newly formed large droplet progressively relaxed to a spherical shape (9–14 min).
E: FRAP assay showing the Stg_CT exchanging kinetics between the condensed droplets and surrounding aqueous solutions. Cy3-labeled Stg_CT (at 30 μM with only 0.5% of Stg_CT Cy3-labeled) was mixed with 10 μM unlabeled PSD-95. The curve represented the averaged signals from 10 droplets with a diameter ~9 μm and the data were plotted as mean ± SD.
F: Representative SDS-PAGE and quantification data of sedimentation experiments showing the distributions of Stg_CT and PSD-95 recovered from the aqueous phase/supernatant (S) and the condensed phase/pellet (P). Proteins were mixed at the indicated concentrations. Results were from 3 independent batches of sedimentation experiments and represented as mean ± SD.
G-H: DIC and fluorescence images showing that the mixtures of (G) 30 μM unlabeled TARP γ−8_CT and 10 μM Alexa 647-labeled PSD-95; or (H) 30 μM Alexa 488-labeled Stg_CT and 10 μM unlabeled SAP102 formed LLPS at room temperature.
See also Figure S1