Abstract
Vulvovaginal candidiasis (VVC), caused by Candida albicans, affects women’s health and life. We aimed to explore the correlation between ERG3 as well as Efg1 mutation/overexpression and azoles-resistance, and the correlation between ERG3 and Efg1 mRNA expression in C. albicans. First, C. albicans was isolated from clinical VVC patients. ERG3 and Efg1 mutations were detected by polymerase chain reaction (PCR) and sequencing, and the expression levels of these two genes were also identified by qRT-PCR. Correlations between mutation/overexpression of ERG3/Efg1 and azoles-resistance as well as ERG3 and Efg1 mRNA expression were analyzed. Based on the ERG3 sequencing, the results showed that there were 2 missense mutation sites, 1 nonsense mutation site, and 4 silent mutation sites, while 1 missense mutation sites, 1 nonsense mutation site, and 12 silent mutation sites were found in Efg1. Furthermore, the mRNA levels of ERG3 gene in the strains sensitive to FCA, ITR or VRC were higher than those in the strains resistant to FCA, ITR, VRC (P < 0.05). While for the mRNA levels of Efg1, susceptible strains were lower than resistant strains. Besides, there was a significant linear negative correlation between ERG3 and Efg1 mRNA expression (r = − 0.614, P < 0.001).
Keywords: Candida albicans, Azoles, Drug resistance, ERG3, Efg1
Introduction
Vulvovaginal candidiasis (VVC), a common disorder in women, is one of the most common infections of the female genital tract [1]. The symptoms of VVC include vaginal itching, burning with urination, white and thick vaginal discharge, pain with sex, and redness around the vagina [2]. It is estimated that 75% of women have had VVC at least once in their lifetime [3]. A primary pathological factor for VVC is Candida albicans strain [4]. At present, azole antifungal drugs, such as voriconazole (VRC), itraconazole (ITR) and fluconazole (FCA), are widely used for the treatment of VVC patients [5]. Unfortunately, the wide use of azole antifungal drugs in clinical leads to azoles-resistant to C. albicans strain, which increases the difficulty of VVC treatment [6]. Therefore, it is needed to find the molecular mechanisms underlying azole resistance, which is significant for the treatment of VVC patients.
Currently, studies for the molecular mechanisms underlying azole resistance of C. albicans are focused on the following four aspects: changes of drug target enzymes, overexpression of efflux pump gene, regulation of zinc-cluster transcription factors, and biofilm formation [7]. Ergosterol, which is an essential component of membrane of C. albicans, influences the activity of membrane-bound enzyme membrane and the membrane permeability [8]. The growth of fungi can be inhibited by anti-fungal azoles via preventing ergosterol synthetic pathway [9]. Erythroblast transformation-specific (ETS) related genes (ERG genes) are targeting enzyme genes in ergosterol synthetic pathway, and over expressions and/or mutations of ERG genes are regarded as the main mechanism of azoles-resistance in C. albicans [10]. Cytochrome P450 lanosterol 14α-demethylase, coded by ERG11 gene, is a target enzyme of azoles [11]. Our research group has confirmed that mutation and/or overexpression of ERG11 leads to azoles-resistance in C. albicans [12, 13]. Furthermore, we have found that overexpression of ERG4 or ERG5 is associated with azoles-resistance in C. albicans [12, 14]. Besides that, there are few studies on other ERG genes in ergosterol pathway at home and abroad. ERG3 (1163 bp), locating in the upstream of ERG11, codes sterol Δ5,6-desaturase, which is a key enzyme in the late stage of ergosterol synthesis [7, 15]. Some studies suggest that mutation of ERG3 gene induces azoles-resistance in C. albicans, and the possible mechanism may be that mutation of this gene prevents the formation of toxic sterol [16, 17]. Akins et al. [18] indicated that overexpression of ERG3 gene increase drug sensitivity of C. albicans. At present, the correlation between ERG3 expression levels and azoles-resistance is not clear, and there is no study on the effects of ERG3 in clinical VVC isolates.
Furthermore, ERG3 is negatively regulated by morphological regulators Efg1 [19]. Efg1, a member of the APSES family, is a major transcription factor in the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) pathway [20]. Efg1 mutation can inhibit the formation of mycelium and decrease the expression of mycelium specific gene, thus reducing the virulence of C. albicans [21]. Efg1 was involved in azoles-resistance of C. albicans and the susceptibility to antifungal drugs increased when lacking this gene [19]. Thus, Efg1 may be associated with azoles-resistance in C. albicans, but the correlation between Efg1 and azoles-resistance is not clear.
C. albicans isolated from VVC patients were used for the present study. ERG3 and Efg1 mutations were detected, and the expression levels of these two genes were also identified. Furthermore, the correlations between mutation/overexpression of ERG3 as well as Efg1and azoles-resistance were analyzed. Besides, the correlation analysis between ERG3 and Efg1 mRNA expression was conducted. We aimed to explore the correlation between ERG3 as well as Efg1 mutation/overexpression and azoles-resistance, and the correlation between ERG3 and Efg1 mRNA expression in C. albicans.
Materials and Methods
Strains
From November 2015 to May 2016, 184 samples of vaginal secretions from patients diagnosed with VVC in department of dermatovenereology, the second hospital of Shanxi Medical University were collected. Among these samples, 50 C. albicans strains were obtained and used for the present study. Approval was obtained from the Ethics Committee of Shanxi Medical University and informed consent was provided by all patients. The reference C. albicans strain, ATCC11006, was purchased from the Fungi and Fungal Disease Research Center of Peking University (Beijing, China).
Drug Susceptibility Testing
The minimal inhibitory concentrations (MICs) of FCA, ITR and VRC was determined by the broth microdilution method supported by the Clinical and Laboratory Standards Institute (CLSI) standard M27–A3. The specific method was carried out by the study of Feng et al. [22]. Results were analyzed according to the CLSI standard: FCA(sensitive, ≤ 8 mg/ml; susceptible dose dependent, 16–32 mg/l; resistant, ≥ 64 mg/l), ITR (sensitive, ≤ 0.125 mg/ml; susceptible dose dependent, 0.25–0.5 mg/l; resistant, ≥ 1 mg/l) and VRC (sensitive, ≤ 1 mg/ml; susceptible dose dependent, 2 mg/l; resistant, ≥ 4 mg/l) [22].
Isolation of Genomic DNA from C. albicans Strains
Genomic DNA was isolated from all C. albicans strains using a Yeast DNAiso kit (Takara Bio, Inc., Otsu, Japan). Briefly, fresh colonies identified as C. albicans were selected and inoculated into liquid nutrient medium and cultured with a speed of 220 rpm at 37 °C overnight. Afterwards, 1 mL fungi suspension was used to extract DNA according to the manufacture’s instruction. The collected DNA was sealed with a sealing membrane and stored at −20 °C for subsequent use.
Polymerase Chain Reaction (PCR) Amplification
According to the gene sequence in GenBank, specific primers of ERG3 and Efg1 (ERG3-F, 5′-ATGGATATCGTACTAGAAATTTGTGA-3′; ERG3-R, 5′-TCATTGTTCAACATATTCTCTATCG-3′; Efg1-F, 5′-ATGTCAACGTATTCTATACCCTATTACAA-3′.
Efg1-R, 5′-TTACTTTTCTTCTTTGGCAACAG-3′) were designed and synthesized by Shanghai Sangon Bioengineering Co. Ltd. (Shanghai, China).
The PCR amplification was performed using 12.5 μL MasterMix (Sangon Bioengineering Ltd, Shanghai, China), 1 μL specific forward primers (10 μmol/L), 1 μL specific reverse primers (10 μmol/L), 40 ng DNA (after dilution), and 9.5 μL ddH2O. The PCR condition was set as denaturation for 5 min at 94 °C, followed by 30 cycles of 94 °C for 30 s, 50 °C (ERG3) or 52 °C (Efg1) for 30 s, and 72 °C for 30 s, and elongation at 72 °C for 8 min and 4 °C for 1 min. PCR products were then separated and sized on a 1.5% agarose (Tianjin No. 3 Chemical Reagent Factory) gel by electrophoresis, and the results were recorded using gel image analyzer to determine whether the amplification was successful or not.
Sequencing and Analysis
The PCR products were purified and sequenced by Shanghai Sangon Bioengineering Co. Ltd. (Shanghai, China). The obtained sequences were compared with the sequence of ERG3 and Efg1 in GenBank using DNASTAR software (Version 7.1), and sequencing map was observed using Chromas (Version 2.6.5) to find point mutations.
Isolation of RNA from C. albicans Strains
Total RNA was isolated as follows: First of all, 1 mL overnight cultured bacterial solution was added to RNAse-free centrifuge tube (1.5 mL), centrifuging at 10,000 rpm for 1 min at room temperature, culture medium was discarded, and thalli (wet weight: 20 mg) was collected. Then, it was washed with 500 µL DEPC-treated ddH2O, centrifuging at 10,000 rpm for 1 min, and supernatant liquid was discarded. A total of 600 µL Snailase Reaction Buffer and 50 µL Snailase (prepared before the experiment) were added to the above tube, water bathing at 37 °C for 5 min, centrifuging at 10,000 rpm for 2 min at 4 °C, and the supernatant liquid was discarded. Then, 400 µL Buffer Rlysis-YS were added immediately, blending by oscillation and water bathing at 65 °C for 5 min. After that, 200 µL Buffer YK were added after ice-bathing for 5 min, centrifuging at 12,000 rpm for 5 min at 4 °C. The supernatant liquid (about 600 µL) was obtained and was added to a new 1.5 mL Rnase-free tube. Equal volume (about 600 µL) of phenol: chloroform (25:24, pH4.5) were added to the supernatant liquid, blending, centrifuging at 12,000 rpm for 5 min at 4 °C. The supernatant liquid was obtained and was added to a new tube, and absolute ethyl alcohol (1/2 volume) was added to the tube, fully blending. The adsorbing column was put into the collection tube, and all the solution was added to the adsorbing column, resting for 1 min, centrifuging at 10,000 rpm for 1 min at room temperature, and the waste liquid in collection tube was poured out. Then, 500 µL RPE Solution was added, resting for 1 min, centrifuging at 10,000 rpm for 1 min at room temperature, and the waste liquid in collection tube was poured out, repeating one time for this operation. Centrifugation was conducted at 12,000 rpm for 2 min at room temperature. The cover of adsorbing column was opened, resting for a few minutes. The adsorbing column was put into a new centrifuge tube, and 30 µL DEPC-treated ddH2O were added to the center of adsorbing membrane, resting for 2 min. Subsequently, it was centrifuged at 12,000 rpm for 2 min, and the obtained RNA was stored at − 70 °C for spare.
Reverse Transcription
Total RNA was reverse-transcribed using Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Shanghai). Firstly, the concentration of total RNA was determined using nucleic acid and protein analyzer. Secondly, PCR reaction was performed as follows: 1 µg RNA and 2 µL OR Random Hexamer Primer were added, and then DEPC-treated water was added until the total volume reached to 13 µL. Denaturation was conducted in PCR instrument at 65 °C for 10 min. Then, it was placed on ice, and 4 µL Transcriptor Reverse Transciptase Reaction Buffer, 0.5 µL Protector RNase Inhibitor, 2 µL Deoxynucleotide Mix, and 0.5 µL Transcriptor Reverse Transciptase were added until the total volume reached to 20 µL. Subsequently, it was put into PCR instrument. Finally, it was taken out from PCR instrument and was stored at − 20 °C for spare.
Design and Synthesis of Primers
According to the cDNA sequence in GenBank, specific primers of ERG3 and Efg1 (ERG3-F, 5′-CGCTTGTCACACTGTCCATC-3′; ERG3-R, 5′- TTCTTCTTCTGCCTTTGCATC -3′; Efg1-F, 5′-GCCACAACCTCAGCATTACA-3′; Efg1-R, 5′-GACCTGGTAGTGGTGGCTGT-3′) were designed and synthesized by Shanghai Sangon Bioengineering Co. Ltd. (Shanghai, China).
Real-Time Quantitative PCR (qRT-PCR) Amplification
The qRT-PCR was conducted using FastStart Essential DNA Green Master (Roche Diagnostics, Shanghai). The reaction system included 10 μL FastStart Essential DNA Green Master, 1 μL specific forward primers, 1 μL specific reverse primers, 1 μL cDNA, and 7 μL ddH2O. The reaction conditions were set as denaturation for 10 min at 95 °C, followed by 45 cycles of 95 °C for 10 s, 55 °C for 10 s, and 72 °C for 10 s, and melting at 95 °C for 10 s, 65 °C for 60 s and 97 °C for 1 s.
ATCC11006 was regarded as the control. The comparative threshold (Ct) cycle method (2−ΔΔCt) was used to calculate relative expression levels of ERG3 and Efg1.
Statistical analysis was performed using SPSS 23.0 software (SPSS Inc., Chicago, Illinois, USA). The results were expressed as X ± SD (means ± standard deviations), and t test was performed. A P value of < 0.05 was regarded as statistically significant difference.
Results
Drug Susceptibility Testing
Drug sensitivity test showed that (Table 1), a total of the 26 strains were sensitive to FCA, 22 strains were FCA-resistant, with a fluconazole resistance rate of 44%. Of the 50 strains isolated, 21 were sensitive to ITR, 1 was susceptible dose dependent and 28 were resistant to ITR, which showed an itraconazole resistance rate of 56%. In addition, the present study identified 23 VRC-sensitive strains, 2 susceptible-dose-dependent strains and 25 VRC-resistant strains, with a voriconazole resistance rate of 50%. Notably, a total of 10 strains were resistant to FCA and ITR, 4 strains were resistant to ITR and VRC, and 12 strains were resistant to all these three drugs. The cross-resistance rates between the three drugs were 20%, 8% and 24%, respectively. The reference strain (ATCC11006) was sensitive to all three drugs [22].
Table 1.
The drug sensitivity test of 50 Candida albicans strains
| Strains (ZY) | MIC (µg/mL) | Strains (ZY) | MIC (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| FCA | ITR | VRC | FCA | ITR | VRC | ||
| 4 | R (64) | R (1) | R (4) | 60 | S (8) | S (0.125) | S (1) |
| 6 | R (64) | R (4) | R (8) | 61 | S (2) | S (0.125) | R (16) |
| 7 | S (2) | R (4) | S (0.5) | 62 | S (4) | S (0.125) | R (8) |
| 8 | R (64) | R (16) | R (8) | 63 | R (64) | R (8) | R (4) |
| 12 | S (1) | S (0.125) | S (0.0125) | 64 | S (0.25) | SDD (0.5) | R (4) |
| 15 | S (0.125) | S (0.0625) | S (0.25) | 66 | S (0.25) | S (0.0625) | S (0.0313) |
| 16 | SDD (16) | R (2) | S (0.5) | 72 | S (0.125) | S (0.0313) | S (0.0313) |
| 18 | R (64) | R (16) | S (0.125) | 77 | R (64) | R (8) | R (16) |
| 19 | R (64) | R (16) | S (1) | 78 | R (64) | R (16) | S (1) |
| 22 | R (64) | R (16) | S (1) | 79 | R (64) | R (2) | S (0.5) |
| 25 | S (8) | S (0.125) | S (0.5) | 80 | SDD (32) | S (0.125) | R (4) |
| 27 | S (8) | S (0.125) | S (0.25) | 88 | S (1) | S (0.0625) | R (8) |
| 28 | R (64) | R (16) | R (8) | 89 | R (64) | R (1) | R (16) |
| 32 | S (0.5) | S (0.0625) | S (0.125) | 90 | S (1) | S (0.125) | S (0.5) |
| 33 | S (4) | S (0.125) | S (0.25) | 91 | R (64) | R (16) | S (1) |
| 41 | S (2) | S (0.0625) | S (0.0313) | 92 | S (0.25) | S (0.0313) | R (4) |
| 44 | R (64) | R (8) | R (4) | 94 | R (64) | R (2) | R (8) |
| 47 | S (0.25) | S (0.0313) | S (0.0313) | 95 | S (8) | R (1) | R (4) |
| 49 | R (64) | R (4) | S (1) | 98 | S (4) | R (2) | R (8) |
| 51 | R (64) | R (2) | S (1) | 99 | R (64) | R (8) | R (16) |
| 53 | R (64) | R (16) | R (8) | 100 | R (64) | R (16) | R (16) |
| 54 | R (64) | R (16) | SDD (2) | 101 | S (8) | R (2) | R (8) |
| 55 | S (1) | S (0.125) | SDD (2) | 105 | S (2) | S (0.125) | R (8) |
| 57 | S (2) | R (1) | R (4) | 163 | S (2) | S (0.0625) | R (4) |
| 59 | R (64) | R (1) | S (1) | 170 | S (0.25) | S (0.0313) | R (8) |
MIC minimum inhibitory concentration, FCA fluconazole, ITR itraconazole, VRC voriconazole, R resistance, S sensitive, SDD susceptible dose dependent
Correlation Between Mutations of ERG3 as Well as Efg1 and Drug Resistance
As presented in Fig. 1, ERG3 and Efg1 were successfully amplified. The ERG3 sequencing results showed that among 50 C. albicans strains, 46 strains were sequenced successfully and 40 strains had base mutations. There were 2 missense mutation sites [C657G (W219C) and C1055T (R352H)], 1 nonsense mutation site [C309T (W103Stop)], 4 silent mutation sites (T342G, T435C, C441T, and T1047C), and 1 termination codon mutated to codon encoding an amino acid [T384C (Stop128 W)]. There was no mutation for standard strain ATCC11006. A total of 3 strains showed missense mutation (2 strains was W219C and 1 strain was R352H), and these 3 strains were FCA/ITR/VRC resistant strains. There was no missense mutation in the susceptible strains (Table 2).
Fig. 1.
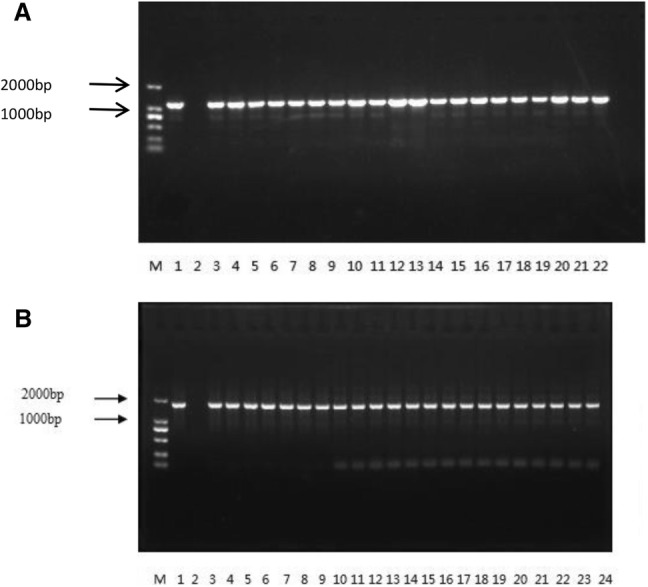
Electrophoretogram of ERG3 (a) and Efg1 (b) in Candida albicans. M: DNA marker; lane1: ERG3 specific amplified fragment of 1161 bp in standard strains; lane 2: blank; lane 3–22: ERG3 specific amplified fragment of 1161 bp in clinical strains. b M: DNA marker; lane1: Efg1 specific amplified fragment of 1653 bp in standard strains; lane 2: blank; lane 3–22: Efg1 specific amplified fragment of 1653 bp in clinical strains
Table 2.
Base mutation sites and amino acid replacement for ERG3 in Candida albicans strains
| Strains (ZY) | Susceptible to FCA/ITR/VRC | Base mutation sites | Amino acid replacement |
|---|---|---|---|
| 4 | FCA/ITR/VRC | T384C/T435C/C441T/T1047C | No |
| 6 | FCA/ITR/VRC | C309T/T435C/C1055Ta | R352H |
| 7 | ITR | C309T | No |
| 8 | FCA/ITR/VRC | C309T | No |
| 12 | S | C309T | No |
| 15 | S | C309T | No |
| 16 | ITR | C309T/T384C | No |
| 18 | FCA/ITR | C309T | No |
| 19 | FCA/ITR | C309T | No |
| 22 | FCA/ITR | C309T | No |
| 25 | S | C309T/T384C/T435C/C441T | No |
| 27 | S | C309T/T435C | No |
| 28 | FCA/ITR/VRC | T342G/T384C/T435C/C441T/C657 Gb | W219C |
| 32 | S | C309T | No |
| 33 | S | C309T | No |
| 41 | S | C309T/T384C/C441T/T435C/T1047C | No |
| 53 | FCA/ITR/VRC | C309T/T435C | No |
| 54 | FCA/ITR | C309T | No |
| 55 | S | C309T | No |
| 57 | ITR/VRC | C309T | No |
| 59 | FCA/ITR | C309T | No |
| 60 | S | C309T | No |
| 61 | VRC | C309T/T384C/T435C/C441T | No |
| 62 | VRC | C309T/T435C | No |
| 63 | FCA/ITR/VRC | T384C/T435C/C441T/T1047C | No |
| 64 | VRC | T384C/T435C/C441T/T1047C | No |
| 66 | S | C309T | No |
| 72 | S | C309T | No |
| 77 | FCA/ITR/VRC | C309T | No |
| 78 | FCA/ITR | C309T | No |
| 79 | FCA/ITR | C309T | No |
| 80 | VRC | C309T | No |
| 89 | FCA/ITR/VRC | T342G/T384C/T435C/C441T/C657 Gb | W219C |
| 90 | S | C309T/T384C/T435C/C441T | No |
| 91 | FCA/ITR | C309T/T435C | No |
| 92 | VRC | C309T/T435C | No |
| 94 | FCA/ITR/VRC | C309T | No |
| 95 | ITR/VRC | C309T | No |
| 163 | VRC | C309T | No |
| 170 | VRC | C309T | No |
W (tryptophan), C (cysteine), R (arginine), H (histidine); FCA/ITR/VRC: resistant to FCA, ITR, and VRC; FCA/ITR: resistant to FCA and ITR; ITR/VRC: resistant to ITR and VRC; S: susceptible to FCA, ITR, and VRC
a,bMissense mutation sites. a: C1055T; b: C657G
The Efg1 sequencing results showed that among 50 C. albicans strains, 40 strains were sequenced successfully and 38 strains had base mutations. There were 1 missense mutation sites [C256T (V86I)], 1 nonsense mutation site [G130A (R44Stop)], 12 silent mutation sites (A150T, A165C, G210A, G267A, G279A, A285T, A744C, A786G, T954A, C1071T, A1055C, and A1317G), and 1 termination codon mutated to codon encoding an amino acid [A1174G (Stop392R)]. There was no mutation for standard strain ATCC11006. A total of 6 strains showed missense mutation, one of which was ITR resistant strain and five of which were FCA/ITR/VRC susceptible strains. There was no V86I mutation for cross resistant strain (Table 3). There were V86I mutations for ITR resistant strain and susceptible strains, and 5 of 16 susceptible strains (mutation rate: 31.3%) and one of 24 resistant strains (mutation rate: 4.2%) showed V86I mutations. Fisher’s exact probability test suggested that there was significant difference for mutation rate between ITR resistant strain and susceptible strains (P < 0.05).
Table 3.
Base mutation sites and amino acid replacement for Efg1 in Candida albicans strains
| Strains (ZY) | Susceptible to FCA/ITR/VRC | Base mutation sites | Amino acid replacement |
|---|---|---|---|
| 4 | FCA/ITR/VRC | A165C/G210A/A1055C | No |
| 6 | FCA/ITR/VRC | G130A/A150T/G210A/G267A/A786G/A1174G | No |
| 7 | ITR | G210A/C256Ta | V86I |
| 12 | S | G210A/C256Ta | V86I |
| 16 | ITR | G130A/A150T/G267A/A1174G | No |
| 18 | FCA/ITR | G210A | No |
| 19 | FCA/ITR | T878G/T954A/C1071T | No |
| 22 | FCA/ITR | G210A/A1317G | No |
| 27 | S | G210A/A786G/T954A | No |
| 28 | FCA/ITR/VRC | G210A/G279A/A285T/A786G/A1174G | No |
| 32 | S | G210A/C256Ta | V86I |
| 33 | S | T878G/T954A/C1071T | No |
| 41 | S | C256Ta/A744C | V86I |
| 44 | FCA/ITR/VRC | G210A | No |
| 47 | S | G130A/A150T | No |
| 49 | FCA/ITR | A150T/G267A/A1174G | No |
| 51 | FCA/ITR | A1174G | No |
| 53 | FCA/ITR/VRC | T878G/T954A/C1071T | No |
| 57 | ITR/VRC | G210A/A1317G | No |
| 61 | VRC | G267A/A1174G | No |
| 62 | VRC | G130A/A150T/G267A/A1174G | No |
| 63 | FCA/ITR/VRC | A1174G | No |
| 64 | VRC | A285T/A786G | No |
| 66 | S | G210A/C256Ta | V86I |
| 72 | S | G210A/C256Ta | V86I |
| 78 | FCA/ITR | C1071T | No |
| 80 | VRC | G210A/A1317G | No |
| 88 | VRC | G210A/A786G/T954A | No |
| 91 | FCA/ITR | A150T/A1174G | No |
| 94 | FCA/ITR/VRC | G210A/A786G/T954A | No |
| 95 | ITR/VRC | G210A/T878G/T954A/C1071T | No |
| 98 | ITR/VRC | T878G/T954A | No |
| 99 | FCA/ITR/VRC | A150T/G267A/A1174G | No |
| 100 | FCA/ITR/VRC | G210A/A1317G | No |
| 101 | ITR/VRC | A150T/A1174G | No |
| 105 | VRC | G210A/A786G/T954A | No |
| 163 | VRC | G210A/A1317G | No |
| 170 | VRC | G210A/A1317G | No |
V (valine), I (isoleucine); FCA/ITR/VRC: resistant to FCA, ITR, and VRC; FCA/ITR: resistant to FCA and ITR; ITR/VRC: resistant to ITR and VRC; S: susceptible to FCA, ITR, and VRC
aMissense mutation site C256T
Correlation Between Expression Levels of ERG3 as Well as Efg1 and Drug Resistance
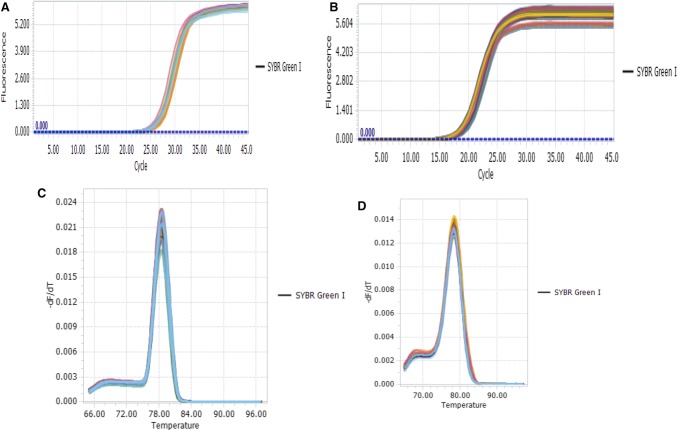
As shown in Fig. 2, the amplification curves showed a typical S-type fluorescence quantitative kinetic curve, and there was only one a single peak at the melting temperature (Tm) and no impurity peak. Target genes of all strains were amplified.
Fig. 2.
The amplification curves and dissolution curves of ERG3 and Efg1. a The amplification curves of ERG3; b The amplification curves of Efg1; c The dissolution curves of ERG3; d The dissolution curves of Efg1
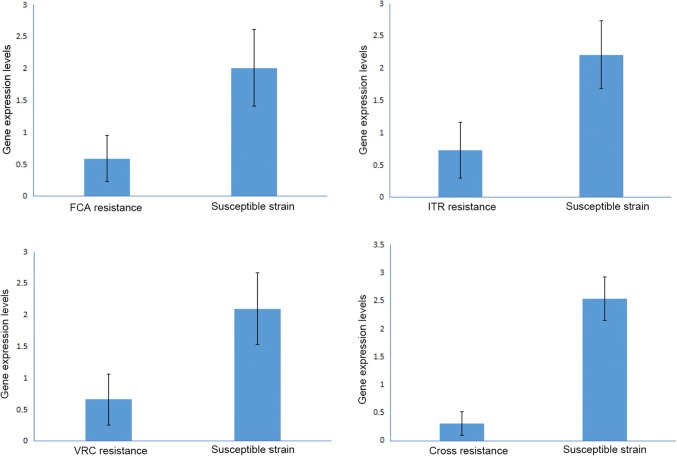
The results of qRT-PCR (Fig. 3) showed that the mRNA levels of ERG3 gene in FCA, ITR, VRC, or cross-susceptible strains were higher than those in FCA, ITR, VRC, or cross-resistant strains (2.01 ± 0.60 vs. 0.59 ± 0.36, P = 0.000; 2.21 ± 0.52 vs. 0.73 ± 0.43, P = 0.000; 2.10 ± 0.57 vs. 0.66 ± 0.40, P = 0.000; 2.54 ± 0.39 vs. 0.31 ± 0.21, P = 0.000).
Fig. 3.
The mRNA levels of ERG3 gene in FCA, ITR, VRC, and cross-resistant strains and the corresponding susceptible strains
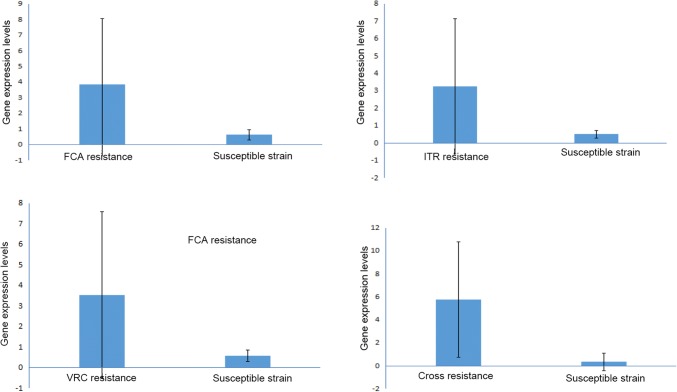
In addition, the results of qRT-PCR (Fig. 4) showed that the mRNA levels of Efg1 in FCA, ITR, VRC, or cross- resistant strains were higher than those in FCA, ITR, VRC, or cross- susceptible strains (3.86 ± 4.20 vs. 0.64 ± 0.32, P = 0.002; 3.26 ± 3.88 vs. 0.51 ± 0.23, P = 0.001; 3.53 ± 4.04 vs. 0.58 ± 0.28, P = 0.001; 5.75 ± 5.01 vs. 0.35 ± 0.76, P = 0.003).
Fig. 4.
The mRNA levels of Efg1 gene in FCA, ITR, VRC, or cross- resistant strains and the corresponding susceptible strains
The results of correlation analysis showed that there was a significant linear negative correlation between ERG3 and Efg1 mRNA expression (r = − 0.614, P < 0.001).
Discussion
Antifungal azole drugs are widely used to treat Candida infections. The molecular mechanisms underlying azole resistance in C. albicans are needed for patient management. Mutations or over-expression in target enzyme genes of ergosterol synthetic pathways (e.g. ERG11) were significantly associated with azoles-resistance in C. albicans [23]. In the present study, the ERG3 sequencing results showed that there were 2 missense mutation sites [C657G (W219C) and C1055T (R352H)], 1 nonsense mutation site [C309T (W103Stop)], 4 silent mutation sites (T342G, T435C, C441T, and T1047C), and 1 termination codon mutated to codon encoding an amino acid [T384C (Stop128 W)], while 1 missense mutation sites [C256T (V86I)], 1 nonsense mutation site [G130A (R44Stop)], 12 silent mutation sites (A150T, A165C, G210A, G267A, G279A, A285T, A744C, A786G, T954A, C1071T, A1055C, and A1317G), and 1 termination codon mutated to codon encoding an amino acid [A1174G (Stop392R)] were found in Efg1. Furthermore, the mRNA levels of ERG3 gene in FCA, ITR, VRC, or cross-susceptible strains were higher than those in FCA, ITR, VRC, or cross-resistant strains, while for the mRNA levels of Efg1, susceptible strains were lower than resistant strains. Besides, there was a significant linear negative correlation between ERG3 and Efg1 mRNA expression.
In the present study, the ERG3 sequencing results showed that 3 strains were missense mutation, 2 strains were W219C and 1 strain was R352H, and these 3 strains were FCA/ITR/VRC resistant strains. Missense mutation was found in FCA/ITR/VRC resistant strains but not in susceptible strains, suggesting that W219C and R352H might be associated with azole resistance. However, the sample size was small and only 3 strains had missense mutation, which might affect the accuracy of the results. One study found that some single mutation, such as D19E, W228Stop, and L266Stop, did not cause azole resistance [24]. Thus, whether single mutations will involve in azole resistance remain to be proved. W219C and R352H were single mutations. To sum up, further studies about the correlation between W219C as well as R352H and azole resistance are needed.
For Efg1 sequencing, 6 strains showed missense mutation [C256T (V86I)], one of which was ITR resistant strain and five of which were FCA/ITR/VRC susceptible strains. There were V86I mutations for ITR resistant strain and susceptible strains, and 5 of 16 susceptible strains (mutation rate: 31.3%) and one of 24 resistant strains (mutation rate: 4.2%) showed V86I mutations. The mutation rate of susceptible strains was higher than that of ITR resistant strains. Furthermore, no V86I mutations were found for FCA/VRC resistant strains. We speculated that V86I mutations might increase the susceptibility of azole. The possible mechanisms might be that the substitution of amino acids led to the change of the spatial structure of the protein encoded by Efg1, affecting the function of protein and even inactivating it, or the substitution of amino acids down-regulated the expression levels of Efg1. However, because of small sample size, fewer mutant strains, and factors other than Efg1, we could not get direct conclusion for the correlation between V86I mutations and azole resistance. Thus, further studies are needed to investigate the relationship between V86I mutations and azole resistance.
A study of Sanglard et al. [25] showed that ERG3 deletion mutations were azole resistant in C. albicans. The possible mechanisms might be as follows: over-expression of ERG3 increased the synthesis of sterol Δ5,6-desaturase, and then more accumulated nontoxic 14α-methylsterol intermediates was transformed into cytotoxic 14α-methylergoster-824 (28)—diene-3 β, 6 α-diol, increasing the synthesis of toxic steroids. As a result, the cell membrane of fungi was destroyed, accelerating their death. Namely, the susceptibility to azole increased. In addition, Akins et al. [18] showed that ERG3 inactivation would confer azole resistance and indicated that wildtype C. albicans strains exposed to azoles typically accumulate the toxic sterol, whereas ERG3 mutants accumulate mostly 14a-methylfecosterol after azole exposure. Another study has showed that the ERG3 expression was increased in FCA-resistant C. parapsilosis and decreased in ITR-resistant and amphotericin B-resistant C. parapsilosis [17]. In our study, the mRNA levels of ERG3 gene in FCA, ITR, VRC, or cross-susceptible strains were higher than those in FCA, ITR, VRC, or cross-resistant strains. Combined with our results, ERG3 mutation might be associated with azoles-resistance in C. albicans, and overexpression of ERG3 was speculated to increase the susceptibility of C. albicans to azoles. However, the mechanisms of ERG3 down-regulation in azole-resistant C. albicans need to be further explored.
Furthermore, the mRNA levels of Efg1 in FCA, ITR, VRC, or cross- resistant strains were higher than those in susceptible strains, and there was a significant linear negative correlation between ERG3 and Efg1 mRNA expression. Prasad et al. [26] indicated that Efg1 level was associated with drug susceptibility in C. albicans. Thus, we speculated that overexpression of Efg1 contributed to azole resistance in C. albicans. The possible mechanisms might be as follows: on the one hand, overexpression of Efg1 affected the content of oleic acid and ergosterol in cell membrane, weakening the membrane fluidity and promoting the formation of biofilm, which strengthened the self-defense system of the strain and weakened the passive diffusion of the drug. As a result, drug resistance of strains increased. On the other hand, overexpression of Efg1 inhibited the expression of ERG3, and lower expression of Efg1 led to drug resistance. Furthermore, one study found that Efg1 deletion strain had higher susceptibility to drugs that targeting ergosterol and its metabolites [19], which also proved this point.
There are some limitations in this study. The W219C and R352H were newly discovered mutation sites in this experiment, but whether the mutation sites will involve in azole resistance remains to be proved. In addition, the correlation between ERG3 and Efg1 as well as azole-resistance should be further explored by gene knockdown or site-directed mutagenesis.
In conclusion, ERG3 mutation was associated with azoles-resistance in C. albicans, and overexpression of ERG3 might increase the susceptibility of C. albicans to azoles. Efg1 mutation might increase the susceptibility to azoles, while overexpression of Efg1 might increase azoles-resistance in C. albicans. Furthermore, there was a significant linear negative correlation between ERG3 and Efg1 mRNA expression in C. albicans. The results in this study may help to improve our understanding of azole-resistant mechanism of C. albicans and design new strategies for antifungal therapy in VVC.
Acknowledgements
This work was supported by Basic Research Project supported by Shanxi Province, China (Program No. 201701D121171), Research and Development Key Projects of Shanxi Province (Program No. 201603D321063), Research Project Supported by Health and Family Planning Commission of Shanxi Province, China (Program No. 201601050). Meanwhile, the project was supported by the Science & Technology Innovation Foundation for Univerisities in Shanxi Province, China (Program No. 20161118).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Wenli Feng and Jing Yang should be regard as co-first authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenli Feng, Phone: +86-0351-3365410, Email: fengwenli@sxmu.edu.cn.
Jing Yang, Phone: +86-0351-3365410, Email: yangjing7962@126.com.
References
- 1.Elfeky DS, Gohar NM, El-Seidi EA, Ezzat MM, Aboelew SH. Species identification and antifungal susceptibility pattern of Candida isolates in cases of vulvovaginal candidiasis. Alex J Med. 2016;52:269–277. doi: 10.1016/j.ajme.2015. [DOI] [Google Scholar]
- 2.Gharaei A, Erahimzadeh A, Khorashad ARS, Jorjani O, Jamshidi A, et al. Determination of prevalancy and species of vulvovaginal candidiasis and clinical findings correlation. J Gorgan Univ Med Sci. 2015;17:109–113. [Google Scholar]
- 3.Matheson A, Mazza D. Recurrent vulvovaginal candidiasis: a review of guideline recommendations. Aust N Z J Obstet Gynaecol. 2017 doi: 10.1111/ajo.12592. [DOI] [PubMed] [Google Scholar]
- 4.Fan SR, Bai FY, Liao QP, Liu ZH, Li J, et al. Genotype distribution of Candida albicans strains associated with different conditions of vulvovaginal candidiasis, as revealed by microsatellite typing. Sex Transm Infect. 2008;84:103–106. doi: 10.1136/sti.2007.025700. [DOI] [PubMed] [Google Scholar]
- 5.Cowen LE, Steinbach WJ. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot Cell. 2008;7:747. doi: 10.1128/EC.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurizio S, Brunella P, Cornelia LFR. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58:2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 7.Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, et al. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol. 2016 doi: 10.3389/fmicb.2016.02173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanglard D, Ischer F, Bille L. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1097/00001813-199802000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46:1704–1713. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perea S, López-Ribot JL, Kirkpatrick WR, Mcatee RK, Santillán RA, et al. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2001;45:2676–2684. doi: 10.1128/AAC.45.10.2676-2684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strzelczyk JK, Slemp-Migiel A, Rother M, Gołąbek K, Wiczkowski A. Nucleotide substitutions in the Candida albicans ERG11 gene of azole-susceptible and azole-resistant clinical isolates. Acta Biochim Polon. 2013;60:547. doi: 10.1016/B978-0-12-420067-8.00023-4. [DOI] [PubMed] [Google Scholar]
- 12.Feng W, Yang J, Xi Z, Qiao Z, Lv Y, et al. Mutations and/or over expressions of ERG4 and ERG11 genes in clinical azoles-resistant isolates of Candida albicans. Microbial Drug Resist (Larchmont, NY) 2017;23:563–570. doi: 10.1089/mdr.2016.0095. [DOI] [PubMed] [Google Scholar]
- 13.Feng W, Yang J, Wang Y, Chen J, Xi Z, et al. ERG11 mutations and upregulation in clinical itraconazole-resistant isolates of Candida krusei. Can J Microbiol. 2016;62:938. doi: 10.1139/cjm-2016-0055. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Feng W, Wang Y, Chen J, Xi Z, et al. Mutation and elevated expression of ERG5 gene in anti-fungal drugs of Candida albicans. China J Health Insp. 2016;4:542–545. [Google Scholar]
- 15.Berkow EL, Manigaba K, Parker JE, Barker KS, Kelly SL, et al. Multidrug transporters and alterations in sterol biosynthesis contribute to azole antifungal resistance in Candida parapsilosis. Antimicrobial Agents Chemother. 2015;59:5942. doi: 10.1128/AAC.01358-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vale-Silva LA, Coste AT, Ischer F, Parker JE, Kelly SL, et al. Azole resistance by loss of function of the sterol Δ5,6-desaturase gene (ERG3) in Candida albicans does not necessarily decrease virulence. Antimicrob Agents Chemother. 2012;56:1960–1968. doi: 10.1128/AAC.05720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotfali E, Ghajari A, Kordbacheh P, Zaini F, Mirhendi H, et al. Regulation of ERG3, ERG6, and ERG11 Genes in antifungal-resistant isolates of Candida parapsilosis. Iran Biomed J. 2017;21:275–281. doi: 10.18869/acadpub.ibj.21.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akins RA. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med Mycol. 2005;43:285–318. doi: 10.1080/13693780500138971. [DOI] [PubMed] [Google Scholar]
- 19.Lo HJ, Wang JS, Lin CY, Chen CG, Hsiao TY, Hsu CT, Su CL, Fann MJ, Ching YT, Yang YL. Efg1 involved in drug resistance by regulating the expression of ERG3 in Candida albicans. Antimicrob Agents Chemother. 2005;49:1213–1215. doi: 10.1128/AAC.49.3.1213-1215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saputo S, Kumar A, Krysan DJ. Efg1 directly regulates ACE2 expression to mediate cross talk between the cAMP/PKA and RAM pathways during Candida albicans morphogenesis. Eukaryot Cell. 2014;13:1169. doi: 10.1128/EC.00148-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su H-c, Cheng B, Shi X-m. The difference of EFG1 and HGC1 expression between the myceial and yeast from of Candida albicans. Chin J Dermatol Vener Dis. 2010;24:304–306. [Google Scholar]
- 22.Feng W, Yang J, Yang L, Li Q, Zhu X, et al. Research of Mrr1, Cap1 and MDR1 in Candida albicans resistant to azole medications. Experimental and Therapeutic Medicine. 2018;15:1217–1224. doi: 10.3892/etm.2017.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casalinuovo IA, Di FP, Garaci E. Fluconazole resistance in Candida albicans: a review of mechanisms. Eur Rev Med Pharmacol Sci. 2004;8:69. [PubMed] [Google Scholar]
- 24.Florent M, Fabrice P, Claire L, Michel M, Patrice LP. Amino acid substitutions in the Candida albicans sterol Δ5,6-desaturase (Erg3p) confer azole resistance: characterization of two novel mutants with impaired virulence. J Antimicrob Chemother. 2012;67:2131–2138. doi: 10.1093/jac/dks186. [DOI] [PubMed] [Google Scholar]
- 25.Sanglard D, Ischer F, Parkinson T, Falconer D, Bille J. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob Agents Chemother. 2003;47:2404–2412. doi: 10.1128/aac.47.8.2404-2412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad T, Hameed S, Manoharlal R, Biswas S, Mukhopadhyay CK, et al. Morphogenic regulator EFG1 affects the drug susceptibilities of pathogenic Candida albicans. FEMS Yeast Res. 2010;10:587–596. doi: 10.1111/j.1567-1364.20. [DOI] [PubMed] [Google Scholar]