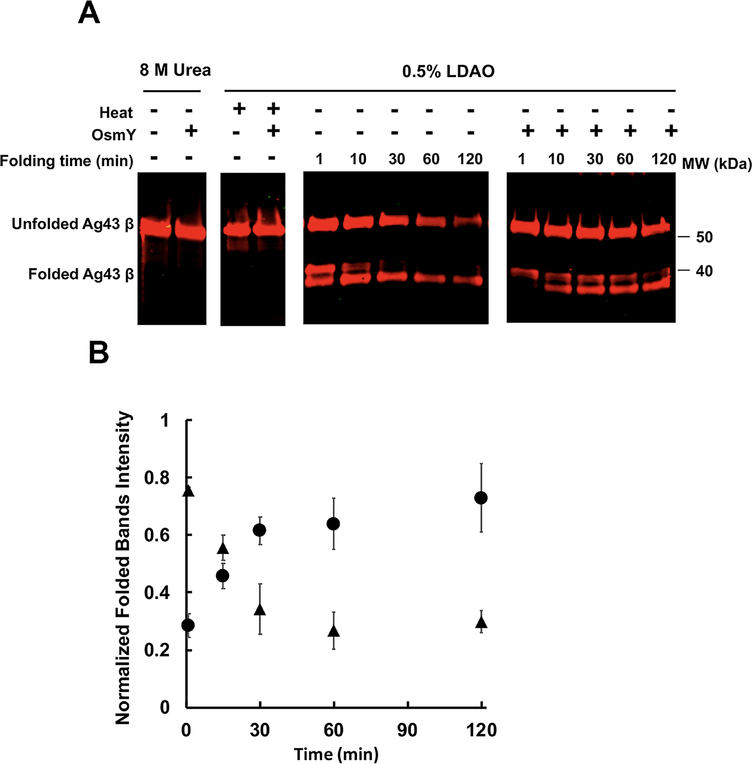
Fig. 3.
Refolding of the Ag43 β domain into LDAO micelles.
A. Purified Ag43 β domain held in 8 M urea was diluted 10 times into 0.5% LDAO buffer to a final concentration of 5 μM at 37°C; buffer either contained 25 μM OsmY or 25 μM BSA. Equal volumes of sample were removed from the reaction mixture at the indicated time points, followed by heating at 95°C for 5 min or not, then analyzed by SDS-PAGE and western blot using antiserum raised against the β domain.
Images are representative of three independent experiments.
B. The folded Ag43 β-domain bands of three independent experiments were quantified using Image J and plotted against time. In each experiment, the intensities of the folded β domain were normalized to urea-denatured unfolded β-domain band intensities, which were first normalized to Ag43 β-domain concentrations used in each experiment. ▲, refolding of β domain without OsmY. ●, refolding of β domain with OsmY.