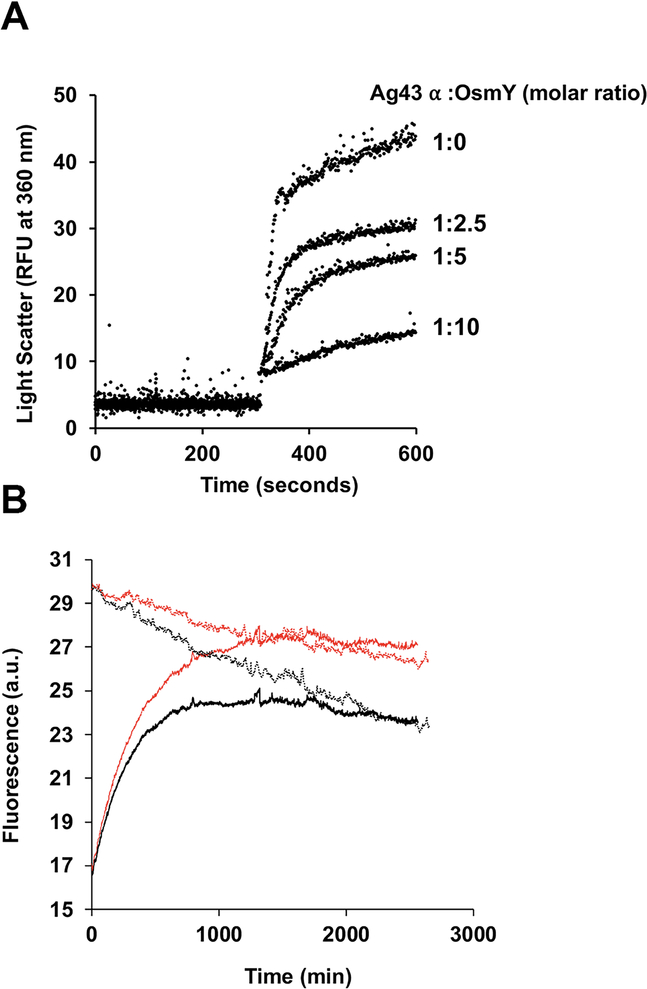
Fig. 5.
OsmY prevents Ag43 α-domain aggregation following acid induced unfolding, but has no effect on its refolding in vitro.
A. 50 μM samples of purified Ag43 α domain in 50 mM Tris pH 8.0, 150 mM NaCl buffer were diluted 20 times into the same buffer at pH 4.0 that had been preincubated with different concentrations of OsmY. Acid induced protein aggregation over time was monitored by measuring light scattering at 360 nm using a fluorescence spectrophotometer at 25°C.
B. 50 μM samples of purified Ag43 α domain were denatured with 8 M urea, and then diluted 50 times into 50 mM Tris pH 8.0, 150 mM NaCl in the presence or absence of 10 μM OsmY. Refolding was monitored by measuring tryptophan fluorescence excitation at 295 nm and emission at 350 nm using a Cary Eclipse fluorimeter at 25°C. Refolding of 1 μM samples of purified Ag43 α domain (native form) was also monitored at the same time using the same refolding assay in the presence or absence of 10 μM OsmY. Solid black, Ag43 α-domain refolding. Solid red, Ag43 α-domain refolding with OsmY. Dotted black, native Ag43 α domain. Dotted red, native Ag43 α domain with OsmY.