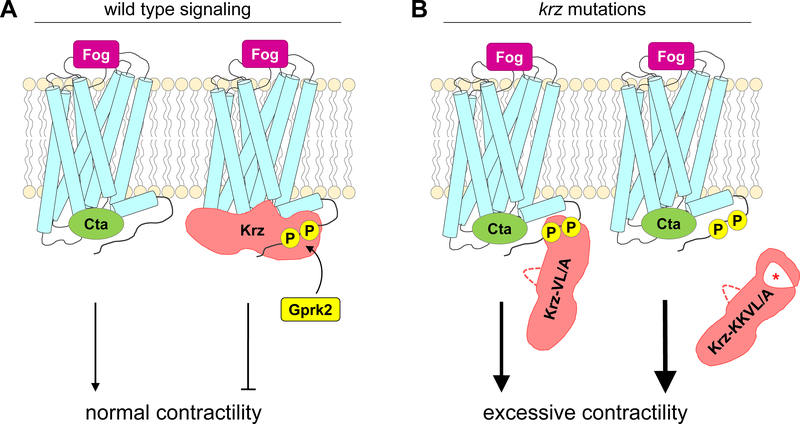
Fig. 5. A model summarizing the effects of the VL/A and KKVL/A mutations on Krz-GPCR interactions and cellular contractility.
(A) In wild type Fog-Mist signaling, the G protein Cta transmits the signal from the Mist receptor activated by its ligand Fog. Mist is then phosphorylated by Gprk2, followed by an interaction with Krz that properly terminates signaling. (B) The finger loop mutation (VL/A) interferes with the ability of Krz to uncouple Mist from Cta, while still allowing partial association with the Gprk2-phosphorylated residues in the receptor tail. Combination of the finger loop and phosphate sensor mutations (KKVL/A) leads to inability of Krz to associate with the receptor. Both the VL/A and KKVL/A mutations result in hyperactivity of the Fog-Mist signaling pathway, leading to excessive cellular contractility.