Abstract
Pulmonary hypertension complicates the care of many patients with chronic lung diseases (defined as Group 3 pulmonary hypertension), yet the mechanisms that mediate the development of pulmonary vascular disease are not clearly defined. Despite being the most prevalent form of pulmonary hypertension, to date there is no approved treatment for patients with disease. Myeloid-derived suppressor cells (MDSCs) and endothelial cells in the lung express the chemokine receptor CXCR2, implicated in the evolution of both neoplastic and pulmonary vascular remodeling. However, precise cellular contribution to lung disease is unknown. Therefore, we used mice with tissue-specific deletion of CXCR2 to investigate the role of this receptor in Group 3 pulmonary hypertension. Deletion of CXCR2 in myeloid cells attenuated the recruitment of polymorphonuclear MDSCs to the lungs, inhibited vascular remodeling, and protected against pulmonary hypertension. Conversely, loss of CXCR2 in endothelial cells resulted in worsened vascular remodeling, associated with increased MDSC migratory capacity attributable to increased ligand availability, consistent with analyzed patient sample data. Taken together, these data suggest that CXCR2 regulates MDSC activation, informing potential therapeutic application of MDSC-targeted treatments.
Keywords: CXCR2, endothelial cell, fibrosis, hypertension, hypoxia, myeloid-derived suppressor cell, pulmonary bleomycin-induced pulmonary
INTRODUCTION
Severe exercise limitation, edema, dyspnea, and a five year survival rate of less than <10%: the storyline is that of an end-stage malignancy but actually describes the course of patients with interstitial lung disease (ILD) and heart failure due to elevated pulmonary artery pressure (12). Unfortunately, pulmonary hypertension (PH), defined as mean pulmonary artery pressure >25 mmHg, affects over half of all patients with the chronic lung disease, including those diagnosed with idiopathic pulmonary fibrosis (IPF; classified as World Health Organization Group 3 PH). As a point of emphasis, Group 3 PH patients have at least a fourfold increased risk of death compared with disease controls without pulmonary vascular disease (31), a similar prognosis to that of newly diagnosed pancreatic cancer. Unfortunately, there are no validated treatments for PH in the setting of interstitial lung disease, except for lung transplant or palliative care, despite a pressing need for such therapies in the clinic.
The parallels between neoplastic growth and Group 3 PH are more than simply extrapolative. Multiple pathways are shared that control both malignant conversion and the aberrant vasculogenesis associated with PH. For instance, the chemokine/chemokine receptor signaling mediated activation of the microenvironmental cellular niche. Along these lines, a dominant hypothesis is that endothelial cell expression of CXC chemokine receptor 2 (CXCR2) is critical in mediating both tumor cell proliferation and the pathogenic capability of lung inflammatory leukocytes in several models of lung disease, including acute lung injury (41), bleomycin-induced pulmonary fibrosis (45), and bronchiolitis obliterans syndrome (3). Of note, CXCR2 is known to be highly expressed in multiple cell types, including endothelial cell precursors, neutrophils, and myeloid-derived suppressor cells (MDSCs). The latter population of cells regulate chronic inflammatory-mediated malignant growth (46), as well as progression of autoimmune disease (50) and an observed correlative relationship with PH (52).
Our group recently demonstrated that the polymorphonuclear subpopulation of MDSCs (PMN-MDSCs) are necessary for the development of PH due to bleomycin-induced pulmonary fibrosis (8). In these studies, mice were protected against development of PH when given a CXCR2 inhibitor, an effect that correlated with a decrease in trafficking of PMN-MDSCs to lungs of treated animals. Based on the murine results, we examined samples from IPF patients and confirmed the pathologic presence of PMN-MDSCs, a validation of the predictive usefulness of the acute bleomycin model. In the cancer literature, cell-type specific expression of CXCR2, and associated signaling ligands, is necessary for proliferation—or arrest—of malignancy (30). The tissue-specific contribution of CXCR2 to PH, in either the endothelial cell or myeloid cell compartment, remains undetermined. Without this knowledge, application of either CXCR2-directed, or MDSC-targeted, therapies will remain out of reach. In the current study, we sought to define the relative role of endothelial-MDSC inflammatory signaling, through CXCR2, in mice undergoing pulmonary vascular remodeling due to bleomycin-associated lung fibrosis and chronic hypoxia exposure.
METHODS
Mice strains and exposures.
VECadherin.Cre (stock 017968), LysM.Cre (stock 004781), CXCR2fl/fl (stock 024638), B6 CD45.1 (002014), and C57BL/6J (stock 000664) were purchased from the Jackson Laboratory. VECadherin.Cre, or LysM.Cre, and homozygous CXCR2fl/fl were crossed to generate either VECaderin.Cre-CXCR2fl/fl (eCXCR2) or LysM.Cre-CXCR2fl/fl (mCXCR2) mice, respectively, for tissue-specific deletion experiments. All mice were older than 10 wk of age at the study onset, included both males and females, and ranged in weight from 20 to 30 g. Mice underwent intraperitoneal injection with 0.018 U/g bleomycin (Thermo Fisher Scientific) or vehicle (PBS) twice weekly for 4 wk (38). Additional animals were exposed to chronic hypoxia in a normobaric chamber (Coy Laboratory Products) with continuous monitoring of oxygen and carbon dioxide concentration. Ventilation was maintained such that carbon dioxide levels remained <0.1%. Mice were housed in the same room under normoxia (room air, : 21%) or hypoxia (: 10%) for a period of 4 wk (6). For MDSC inhibition studies, anti-Gr1 antibody (clone RB6–8C5) and isotype control (clone LTF2), both purchased from BioXcell, were dosed at 200 μg/mouse (intraperitoneal) once weekly upon initiation of bleomycin protocol (49).
Pulmonary hemodynamic and histologic assessments.
Upon completion of experimental protocols, intact mice underwent invasive closed-chest measurement of right ventricular systolic pressure (RVSP). In brief, a Millar 1.4-French pressure-volume microtip catheter transducer (SPR-839; Millar Instruments) connected to a PowerLab/8s (ADInstruments) was inserted through a right internal jugular vein incision and threaded down into the right ventricle. RVSP (mmHg) recordings were collected using Chart 5 (ADInstruments). Upon completion of the measurements, the heart was excised with removal of the atria, and the RV and left ventricle (LV) plus septum (LV+S), isolated for measurement of the RV:LV + S as previously described (17). Formalin-fixed, paraffin-embedded, lung tissue was then assessed for semiquantitative fibrosis score, as previously described (29). Lung histology was additionally stained for α-smooth muscle actin and assessed for muscularized pulmonary vessel count (7). Images were obtained using a Keyence BZ-X microscope, with analysis performed using BZ-X Analyzer software (Keyence).
Adoptive transfer protocol.
For experiments of MDSC adoptive transfer, MDSCs were isolated from femurs and blood of bleomycin/clodronate liposome-treated B6 CD45.1 mice by Myeloid-Derived Suppressor Cell Isolation Kit (Miltenyi Biotec) after lysis of red blood cells according to the manufacturer’s instructions. Unstimulated CD45.1 mouse tissue was used for neutrophil control injection experiments. Isolated MDSCs were then aliquoted, and 1 × 106 of purified MDSCs were intravenously injected into recipient C57BL/6J mouse once a week from the beginning of bleomycin treatment to the end of the experimental protocol (24).
T-cell proliferation assay.
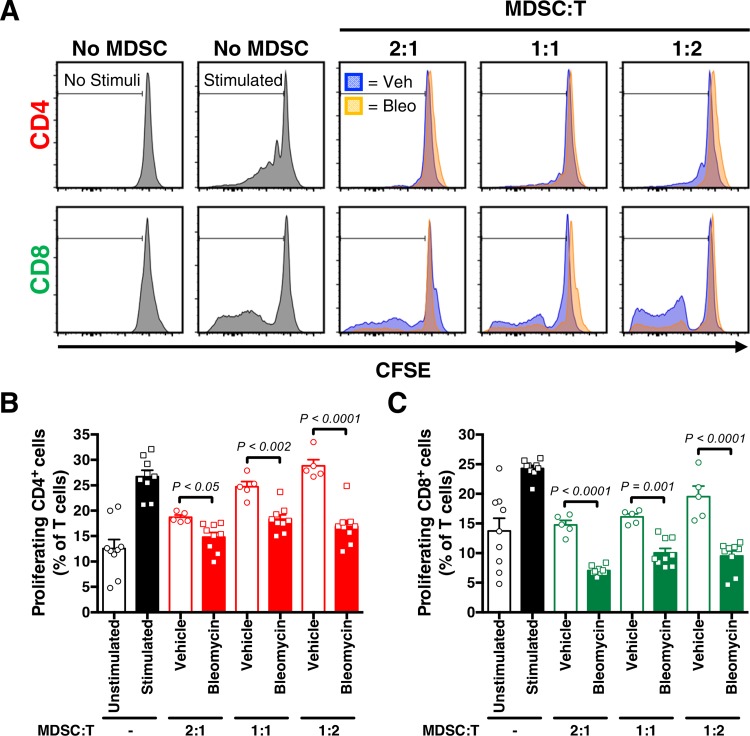
T-cell proliferation/suppression assay was performed as previously described (18). In brief, wild-type C57BL/6 T cells were isolated with CD3 monoclonal antibody-coated magnetic beads (Miltenyi Biotec) and stained with CFSE. These cells were then stimulated with anti-CD3/CD28 mouse beads (ThermoFisher). The ratio between MDSCs and T cells was tested at 2:1, 1:1, and 1:2. Cells were incubated for 5 days, with proliferation assessed as CFSE dilution by flow cytometry and percent proliferation calculated for each group.
Endothelial cell isolation.
Primary isolates of pulmonary microvascular endothelial cells (PMVECs) were obtained from transgenic mice and littermate controls, as previously described (6). In brief, cells were prepared from uninjured mice using collagenase type 2 and red blood cell lysis buffer. Endothelium was then isolated by fluorescence-activated cells sorting based on CD31/PECAM-1 expression. To induce endothelial differentiation, sorted cells were plated on gelatin-coated plastic and were cultured in endothelial growth medium (Lonza), until cells were confluent. Cells were then incubated with Alexa Fluor 488-labeled AcDiLDL (Life Technologies), and positively stained cells were enriched by flow cytometry. Patient explanted lungs rejected for transplant were used for healthy controls, in addition to primary purchased cells (Lonza).
Statistical analysis.
All graphing and statistical analyses were carried out using GraphPad Prism (GraphPad Software). All animal data are presented as means ± SE. The Student’s t-test was used for single comparisons, and two-way ANOVA was used for multiple comparisons. Human data are presented as median ± interquartile range. The Mann-Whitney U-test or the Kruskal-Wallis rank-sum test was used for nonnormal data, with Holm-Sidak’s multiple comparison test. P < 0.05 was considered significant.
Study approval.
All animal studies were approved by the University of Florida Institutional Animal Care and Use Committee. Additionally, human sample use was approved by the Institutional Review Board of both the University of Florida and Rhode Island Hospital. Regarding the latter cohort, subjects were enrolled from the Rhode Island Hospital Pulmonary Hypertension Center as part of a local registry and biorepository (4), which captures all patients referred for PH evaluation. Subjects were included if they were clinically phenotyped as pulmonary fibrosis [IPF defined by ATS/ERS 2011 guidelines (40)] with no PH or pulmonary fibrosis with PH, assessed by standard right heart catheterization hemodynamic parameters and echocardiographic criteria (48). Exclusion criteria included history of chronic thromboembolic pulmonary hypertension, left heart failure, obstructive sleep apnea, and history of congenital heart disease.
The online Supplemental Data (available at https://doi.org/10.6084/m9.figshare.7926830) provide supplemental figures and further details of the animal studies, MDSC adoptive transfer, T-cell proliferation assay, endothelial cell isolation with described in vitro analysis, human patient characteristics, and statistical analysis.
RESULTS
Patients with interstitial lung disease and pulmonary hypertension display increased CXCR2 expression in circulating myeloid cells, decreased in pulmonary vascular endothelial cells.
Previously our group defined an increase in MDSCs in the circulation of patients with ILD, including those diagnosed with IPF and chronic hypersensitivity pneumonitis (8), expanding upon earlier investigators’ work examining MDSCs in the blood of patients with IPF (15). Reasoning from what is known in studies of malignant growth, we hypothesized that the amount of CXCR2 on myeloid cells would be increased in a separate cohort of patients with ILD and PH, inclusive of patients with IPF and non-IPF ILD (18). We analyzed, by flow cytometry, cells from peripheral blood in three patient cohorts: healthy controls (HC) and patients with parenchymal lung disease, with (ILD + PH) and without PH (ILD; characteristics detailed in materials and methods), noting an elevation of MDSCs (CD11b+CD33+HLA-DR− cells) in the latter two disease groups as anticipated (Fig. 1A). We next characterized MDSC subpopulations within groups and found an overall decrease in the percentage of monocytic MDSCs (CD11b+CD33+HLA-DR−CD14+ cells; Mo-MDSCs, Fig. 1B), with a corresponding increase in PMN-MDSCs (CD11b+CD33+HLA-DR−CD14−CD15+ cells, Fig. 1C), in those patients with ILD compared with controls (Fig. 1, A and B). The PMN-MDSC expression of CXCR2, assessed by mean fluorescent intensity (MFI), was increased in patients with lung disease and PH versus those without PH and HC, while there were no differences among ILD cohorts in the chemokine receptor expression by Mo-MDSCs (Fig. 1, D and E).
Fig. 1.
Patients with interstitial lung disease (ILD), including idiopathic pulmonary fibrosis (IPF) and pulmonary hypertension (PH), display augmented expression of CXCR2 in myeloid-derived suppressor cells (MDSCs) and pulmonary microvascular endothelial cells (PMVECs). A: changes in percentage of live CD11b+CD33+HLA-DR− MDSCs in healthy controls (HC; n = 7) and ILD with (n = 6) and without PH (n = 8) peripheral blood samples. B and C: differences in percentage of CD11b+CD33+HLA-DR−CD14+ cells (monocytic MDSCs, Mo-MDSCs) and CD11b+CD33+HLA-DR−CD14−CD15+ cells (polymorphonuclear MDSCs, PMN-MDSCs) between HC and ILD groups. D and E: expression of CXCR2 (mean fluorescence intensity; MFI) in Mo-MDSCs and PMN-MDSCs between group samples. F–I: immunofluorescent staining and signal intensity quantification for interleukin-8 (IL-8), CD31, and CXCR2 in histologic lung samples from healthy controls (HC; n = 6) and IPF patients with (n = 5) and without (n = 6) PH (×4; scale bar 500 μm). J: PMVECs isolated from HC (n = 3), IPF (n = 16), and IPF with PH (n = 9) patients’ lungs with expression, by RT-PCR, of CXCR2 exposed to normoxic (Nx) or hypoxic (Hx; 1% O2) conditions. K: concentration (pg/mL) of IL-8, by ELISA, in conditioned media of isolated groups’ PMVECs exposed to Nx or Hx. All flow data are presented as median ± interquartile range, and immunofluorescent and molecular analysis is presented as means ± SE. The Mann-Whitney U test or the Kruskal-Wallis rank-sum test was used for nonnormal data comparison. P < 0.05 was considered significant.
It is known that in patients with IPF peripheral circulatory and bronchoalveolar lavage fluid IL-8 levels, the human ligand for CXCR2, are elevated (53) and associated with worsened mortality (43) related to increased angiogenic activity (25). Therefore, we hypothesized that IL-8/CXCR2-axis signaling would be altered in lung samples from a separate, previously described (8), group of patients with IPF and PH (patient samples provided through the National Institutes of Health-Lung Tissue Research Consortium; NIH-LTRC). First, we noted that IL-8 fluorescent signal intensity was globally decreased in lung sections from the patient cohort with IPF and PH (Fig. 1F), compared with disease and healthy controls. This decrease in ligand fluorescent signal correlated with a significant reduction in vascular endothelial CXCR2 expression (as indicated by costaining of CXCR2 with CD31, Fig. 1, G–I). While a decrease in vascular receptor protein may be influenced by a decrease in the density of the vascular bed itself, we found no difference in CD31 (endothelial cell) signal intensity between patient group samples, consistent with prior reports (23).
Finally, to explore more fully any cell autonomous CXCR2/IL-8 differences between groups, we isolated PMVECs from explants of another group of patients with IPF with or without PH (patient characteristics in Supplemental Table S1) who underwent lung transplantation at our institution, comparing cellular response to hypoxic stimuli between groups. Endothelial cells from patients with IPF and PH displayed reduced expression of CXCR2 by real-time PCR (Fig. 1J), while IL-8 in conditioned media was elevated compared with normoxic healthy and disease controls, with no difference in response to hypoxia (Fig. 1K). Cellular expression of associated matrix remodeling proteins matrix metallopeptidase 9 (MMP9) and tissue inhibitor matrix metalloproteinase 1 (TIMP1), without significant change in homologous MMP2 or MMP7, was deranged in samples from patients with PH, as well (Supplemental Fig. S1, A–D). Collectively, these data demonstrate that patients with pulmonary fibrosis and PH display increased PMN-MDSC CXCR2 and decreased pulmonary vascular endothelial cell CXCR2, with increased IL-8 signaling in vitro, associated with excess ligand present in microenvironment compared with disease controls.
MDSCs directly mediate pulmonary vascular remodeling.
Before we could explore the cell-specific nature of CXCR2 in development of PH, we first had to define the MDSC population contribution in our disease models. Intraperitoneal administration of bleomycin causes chronic low-grade lung injury in mice, which induces pulmonary fibrosis and hypertension, associated with an accumulation of MDSCs within the lung (47). Therefore, to appropriately interpret further studies using this model of lung injury, it was necessary to first demonstrate that isolated MDSCs from bleomycin-treated mice, used to induce inflammatory lung injury and fibrosis, have the ability to suppress T-cell proliferation. Using a T-cell suppression assay, we demonstrate that MDSCs from mice treated with chronic bleomycin display an expected decrease in T-cell proliferation, in a dose-response manner according to cocultured MDSC concentration (CD4+ and CD8+ T proliferation using CFSE marker compared with vehicle-treated controls; Fig. 2, A–C).
Fig. 2.
Myeloid-derived suppressor cells (MDSCs) suppress T-cell proliferation in vitro. A–C: representative histograms and proliferation index (CD4+ and CD8+ cells, as % of T cells) after MDSCs purified from spleens of vehicle (Veh) or bleomycin-treated (Bleo) wild-type mice were cultured with T cells labeled with CFSE, stimulated with anti-CD3/CD28 antibodies, at different ratios. n = 5–9 Mice/group. All data are presented as means ± SE. Student’s t test was used for single comparison analysis. P < 0.05 was considered significant.
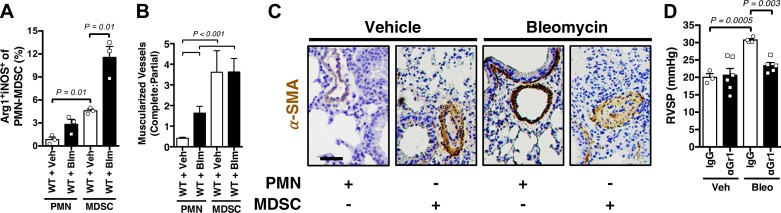
Based on our prior observed association between lung MDSC stimulation and development of PH, we next assessed the ability of simple MDSC adoptive transfer to augment early bleomycin-induced pulmonary vascular remodeling (graphic summary figure for adoptive transfer protocol and demonstration of adoptive cell detection by flow cytometry displayed in Supplemental Fig. S2, A and B). First, we found that, compared with control mice receiving unstimulated neutrophil adoptive transfer, mice that received MDSC transfer had an increase in lung arginase 1- and inducible nitric oxide synthase-positive PMN-MDSCs, consistent with a MDSC signature and inconsistent with activated/mature neutrophil markers (Fig. 3A). Second, we found a significant increase in the ratio of completely muscularized to partially muscularized pulmonary vessels in both the vehicle and bleomycin-treated MDSC-injected mice, as compared with controls (assessed via α-smooth muscle actin muscularized vessels counts; Fig. 3, B and C). Finally, we showed that treatment with an inhibitor of MDSC activation resulted in an expected increase in the absolute number of PMN-MDSCs in the lung (42) (Supplemental Fig. S2C), while mice were protected against development of bleomycin-associated PH (assessed by invasive closed-chest measurement of right ventricular systolic pressure; Fig. 3D). Cumulatively, these data are consistent with a definitively causative role for MDSCs in mice undergoing pulmonary vascular remodeling.
Fig. 3.
Myeloid-derived suppressor cells (MDSCs) cause pulmonary vascular remodeling. A: percentage (%) of arginase 1 (Arg1) and inducible nitric oxide synthase (iNOS)-positive granulocytic (PMN-MDSCs) in MDSCs versus neutrophil (PMN)-treated wild-type (WT; C57BL/6J) vehicle (Veh) and bleomycin (Bleo) mice. B: complete:partial muscularized pulmonary vessel ratio in treated groups. C: representative α-smooth muscle actin (α-SMA; brown)-stained pulmonary vessels in treated groups, at low magnification (×20; scale bar 100 μm). D: right ventricular systolic pressure (RVSP) in IgG or anti-Gr1 (RB6-C85), MDSC inhibited, WT mice exposed to Veh or Bleo. n = 3–6 Mice/group. All data are presented as means ± SE. Student’s t test was used for single comparisons. P < 0.05 was considered significant.
Myeloid cell expression of CXCR2 is necessary for MDSC-mediated pulmonary vascular remodeling.
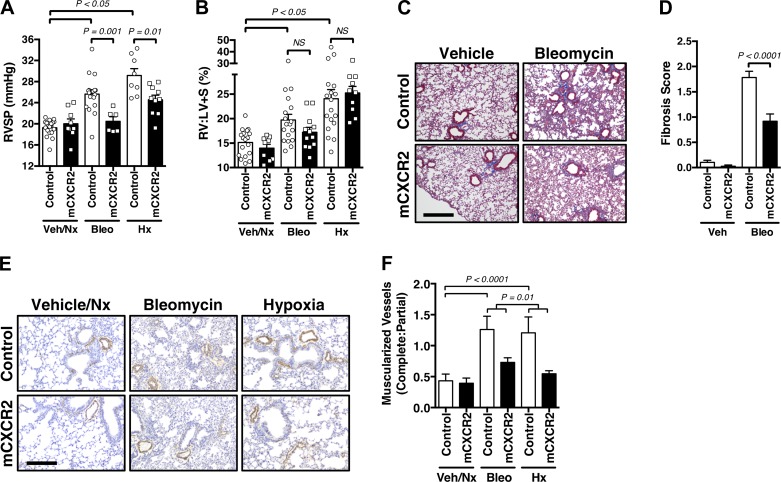
Subsequently, to better understand the mechanism of CXCR2 contribution to pulmonary vascular remodeling, and given the primary role for MDSCs in maladaptive vascular changes of the lung, we next investigated the effects of myeloid cell CXCR2 deletion on PH. To this end, we developed mice with CXCR2 deleted in myeloid lineage tissue, which includes MDSCs (14), by crossing LysM.Cre with CXCR2fl/fl mice, to generate LysM.Cre-CXCR2fl/fl transgenic animals (hereafter referred to as mCXCR2 mice). First, the mCXCR2 mice were nearly completely protected against the development of PH in response to bleomycin and chronic hypoxia exposure, as measured by RVSP (Fig. 4A), without change in RV remodeling (Fig. 4B), suggesting a primarily vascular intrinsic change. This phenotype was associated with an overall decrease in whole lung MDSC populations expressing CXCR2 (without major changes observed in alveolar/interstitial macrophage or neutrophil lung populations of treated groups; Supplemental Fig. S3, A–D), consistent with CXCR2-mediated activation and recruitment of noted cells to the lungs.
Fig. 4.
Lack of myeloid cell CXCR2 attenuates myeloid-derived suppressor cell (MDSC)-mediated pulmonary vascular remodeling. A: right ventricular systolic pressure (RVSP; mmHg) of control and LysM.Cre-CXCR2fl/fl (mCXCR2) mice exposed to vehicle (Veh)/normoxia (Nx), bleomycin (Bleo), or hypoxia (Hx). B: right ventricle-to-left ventricle plus septum mass ratio (RV:LV + S; %) in treated groups. C and D: representative Masson’s trichrome stain and semiquantitative fibrosis score illustrating degree of pulmonary fibrosis in control versus mCXCR2 mice, either Veh and Bleo exposed (×10; scale bar 150 μm). E: representative α-smooth muscle actin (brown)-stained pulmonary vessels in treated groups, at low magnification (×10; scale bar 150 μm). F: complete:partial muscularized pulmonary vessel ratio in treated groups. n = 6–18 Mice/group. All data are presented as means ± SE. Student’s t test was used for single comparisons, and 2-way ANOVA was used for multiple comparisons. P < 0.05 was considered significant.
Additionally, mCXCR2 mice were found to be protected against development of bleomycin-induced pulmonary fibrosis (Fig. 4, C and D) and bleomycin-induced pulmonary vessel muscularization, as assessed by semiquantitative modified fibrosis score (Masson’s trichrome stain; Fig. 4, E and F). These data are consistent with a prior report noting that a CXCR2 inhibitor was protective against the development of bleomycin-induced pulmonary fibrosis in mice (45). In total, these experiments support a direct causative role for CXCR2 expressed by myeloid cells, including MDSCs, in the pathogenesis of PH.
Endothelial cell expression of CXCR2 is protective against development of pulmonary vascular remodeling.
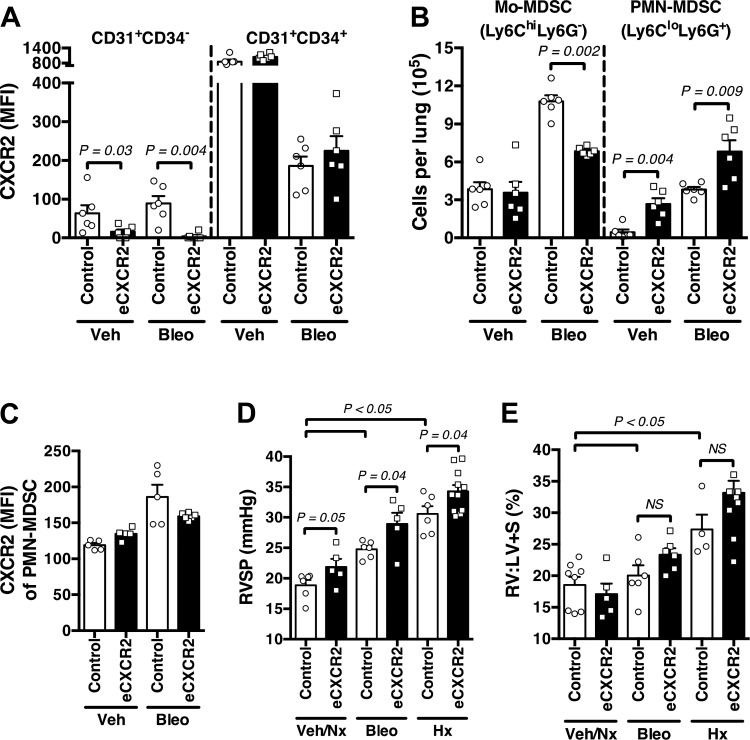
To date, endothelial cell expression of CXCR2 has been assumed to be the primary contributor to PH development (9, 10) and not leukocyte-specific chemokine receptor expression. Therefore, to fully examine these vasculopathic changes due to endothelial cell-specific expression of CXCR2, we developed transgenic animals with vascular endothelial deletion of the chemokine receptor (VECadherin.Cre-CXCR2fl/fl; hereafter referred to as eCXCR2 mice). First, upon comparison of lung samples between vehicle and bleomycin-exposed groups by flow cytometry (gating strategy in Supplemental Fig. S3A), eCXCR2 mice had little to no expression of CXCR2 on mature endothelial cells (MFI; assessed on CD45−CD31+CD34− gated cells) compared with littermate controls (Fig. 5A), as expected. Conversely, CD45−CD31+CD34+ endothelial progenitor cells, known to express high levels of CXCR2 for cellular homing (20), expressed equal CXCR2 among control and eCXCR2 mice, demonstrating relative specificity of the deletion to mature endothelial cells, an internal experimental positive control.
Fig. 5.
Endothelial CXCR2 depletion heightens myeloid-derived suppressor cell (MDSC)-mediated pulmonary vascular remodeling. A: CXCR2 expression (mean fluorescence intensity; MFI) in mature endothelial cells (CD45−CD31+CD34−; EC) and endothelial progenitor cells (CD45−CD31+CD34+) in control and VECadherin.Cre-CXCR2fl/fl (eCXCR2) mice exposed to vehicle (Veh) or intraperitoneal bleomycin (Bleo). B: absolute number of monocytic-MDSCs (Mo-MDSCs) and PMN-MDSCs in lungs of control versus eCXCR2 mice exposed to Veh or Bleo. C: CXCR2 expression by PMN-MDSCs in control and eCXCR2 mice exposed to Veh or Bleo. D: right ventricular systolic pressure (RVSP; mmHg) of control and eCXCR2 mice exposed to Veh/normoxia (Nx), Bleo, or hypoxia (Hx; 10% ). E: right ventricle-to-left ventricle plus septum mass ratio (RV:LV + S; %) in treated groups. n = 6–8 Mice/group. All data are presented as means ± SE. Student’s t test was used for single comparisons, and 2-way ANOVA was used for multiple comparisons. P < 0.05 was considered significant.
Next, we found a significant increase in both Mo-MDSC and PMN-MDSC subpopulations within the lungs of control mice exposed to bleomycin, with eCXCR2 mice displaying a blunted increase in Mo-MDSCs, and a concurrent exaggerated expansion of PMN-MDSCs, compared with controls (Fig. 5B). Because VECadherin is expressed within the bone marrow of adult mice (1), it was necessary to also confirm that PMN-MDSCs from these mice express CXCR2, comparable to control mice upon bleomycin exposure (Fig. 5C), which they do as determined by MFI on flow cytometric analyses. Regarding physiologic changes, eCXCR2 mice were not protected against worsening pulmonary vascular remodeling, displaying a significant increase in right ventricular systolic pressure (RVSP; mmHg), compared with control mice, in response to both bleomycin-induced pulmonary fibrosis and chronic hypoxia exposure, itself known to stimulate MDSC recruitment (22). Again, there was no change in right ventricular remodeling by the right ventricle-to-left-ventricle plus septal mass ratio (RV:LV + S; %) (Fig. 5, D and E), favoring an evolution of lung vascular changes instead. Regarding fibrosis, eCXCR2 mice showed increased lung fibrosis compared with controls, as well as mCXCR2, as determined by fibrosis score and whole lung expression of type 1 collagen (COL1A1) and fibronectin, by RT-PCR (Supplemental Fig. S4, B–E). Of note, as opposed to previously noted decrease in fibrosis assessed by modified semiquantitative scoring, there was no significant difference between control and mCXCR2 samples in extracellular matrix protein expression. Notably, however, there was no evidence of pulmonary vessel dropout in the lungs of eCXCR2 bleomycin mice (Supplemental Fig. S4, F and G), consistent with the aforementioned well-described lack of relationship between severity of lung fibrosis and development of PH (2, 44). Cumulatively, these data demonstrate a heretofore unappreciated protective role for endothelial CXCR2 in the pulmonary vascular response to noxious stimuli.
CXCR2 deletion on pulmonary microvascular endothelial cells increases MDSC trafficking and activation.
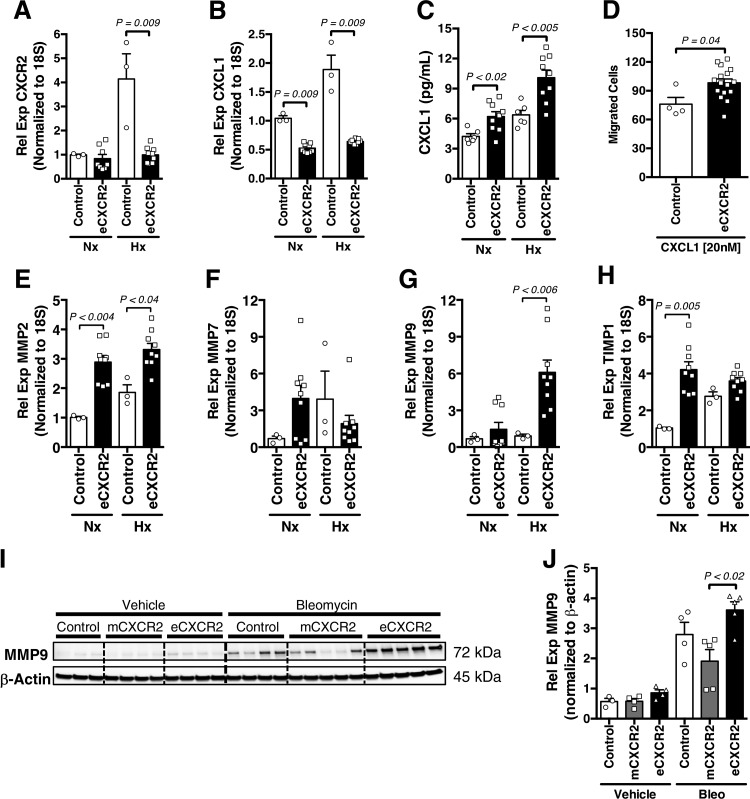
Together, the preceding observations demonstrate that deficiency of CXCR2 in the vascular endothelium of mice results in a dramatic increase in whole lung PMN-MDSC accumulation associated with pulmonary vascular remodeling. Furthermore, it is known that if CXCR2 is sterically inhibited on endothelial cells, ligand CXCL1 production is equally arrested (37). Therefore, we reasoned that since the major mechanism of clearance of chemokine ligands from the tissue microenvironment is receptor-mediated uptake (11), deletion of CXCR2 on endothelial cells may result in increased tissue levels of CXCR2 ligands. We consequently examined the expression of CXCL1 in isolated pulmonary microvascular endothelial cells (PMVECs) from eCXCR2 mice exposed to either normoxia or hypoxic stimulus. Upon confirming the lack of CXCR2-expression in stimulated eCXCR2 PMVEC (Fig. 6A), we discovered that despite a compensatory expected decrease in transcription level expression of CXCL1 by cells from transgenic animals, CXCL1 protein was increased in the conditioned media of hypoxic PMVECs (Fig. 6, B and C). This phenomenon, consistent with decreased ligand uptake by CXCR2 in PMVECs lacking the receptor, may have contributed to the increase in MDSC migration toward a CXCL1 gradient in the presence of eCXCR2 versus control PMVEC (using a modified Boyden chamber; Fig. 6D).
Fig. 6.
Pulmonary microvascular endothelial cells (PMVECs) from mice without endothelial CXCR2 expression (VECadherin.Cre-CXCR2fl/fl; eCXCR2) elicit increased myeloid-derived suppressor cell (MDSC) migration in response to preserved chemokine gradient. A and B: RT-PCR expression of both CXCR2 and its ligand CXCL1 in PMVECs isolated from eCXCR2 mice exposed to normoxic (Nx) or hypoxic (Hx; O2 1%) conditions. C: concentration (pg/mL) of CXCL1, by ELISA, in conditioned media of control versus eCXCR2 mice isolated PMVEC exposed to Nx or Hx. D: MDSC migration in response to coculture with either control or eCXCR2 PMVECs, spiked with CXCL1 (20 nM). E–H: RT-PCR expression of matrix metallopeptidase 2 (MMP2), MMP7, MMP9, and tissue inhibitor matrix metalloproteinase 1 (TIMP1), respectively, in PMVECs isolated from eCXCR2 mice exposed to Nx or Hx. I and J: immunoblot of MMP9 (normalized to β-actin) and graph of densitometry quantification in whole lung of vehicle or bleomycin-exposed control, LysM.Cre-CXCR2fl/fl (mCXCR2), and eCXCR2 mice. n = 3–8 Mice/group. All data are presented as means ± SE. The Student’s t test was used for single comparisons, and 2-way ANOVA was used for multiple comparisons. P < 0.05 was considered significant.
Changes in chemokine receptor density can lead to decline in the overall health of PMVECs isolated from the eCXCR2 mice, with a noted decrease in endothelial cell migratory capacity, angiogenesis capability, and barrier permeability in isolated endothelial cells (as determined by transendothelial electrical resistance measurements of isolated transgenic and control-derived cells) (Supplemental Fig. S5, A–D). Associated with these observations, extracellular matrix proteases are produced by a variety of cell types in the lung, with endothelial cell CXCR2 signaling playing a necessary role for coordinated angiogenesis and vascular permeability in response to injury (32). To this end, although MMP7 endothelial cell expression remained unchanged between exposures and genotypes, we found a significant increase in MMP2, MMP9—and its associated tissue-level inhibitor TIMP1—in normoxia or hypoxia-treated eCXCR2 cells (Fig. 6, E–H). The latter observation mirrored our patient isolated endothelial cell data. Pursuing this further, the relatively MDSC-specific changes in the lung of our transgenic mice were supported by the increase in whole lung protein levels of MMP9, a penultimate common mediator for relevant disease states including PH (51), pulmonary fibrosis, (13), and cell and molecular signaling including MDSC (36) and CXCR2/CXCL1 signaling (19) in bleomycin-treated eCXCR2 whole lung samples, compared with both controls and mCXCR2 mice lungs (Fig. 6, I and J). These changes are consistent with the enhanced MDSC recruitment capability of isolated PMVECs from eCXCR2 mice and support a potential mechanism of contribution from cell-cell cross talk to extracellular matrix changes in PH development.
DISCUSSION
These results illustrate several mechanisms that reveal the complexity of myeloid cell recruitment to the lung and subsequent development of PH. Furthermore, these data highlight several novel aspects of disease that need to be considered in therapy development, especially when exploring combinations of medicines used in routine treatment of either IPF or PH, that may directly or indirectly act on MDSC trafficking, activation, and proliferation. For example, previous studies have demonstrated that CXCR2 signaling drives progression of PAH (9, 10). However, because chemokine receptor-mediated activities have pleiotropic effects, depending on cell-type expression, it has remained unclear which major tissue-bed expressing CXCR2 contributes to disease. Similar to data from the existing cancer literature, studies have confirmed the angiogenic function, and autologous role, of endothelial CXCR2 and human ligand IL-8 (33). However, to date, no study has implicated CXCR2 activation by MDSC subsets in development of pulmonary vascular remodeling, leading to PH. Using multiple in vivo models of PH, we investigated the function of myeloid and endothelial cell CXCR2 expression in the lung vascular response to chronic injury. Surprisingly, in contrast to myeloid, and MDSCs, specifically, CXCR2 expression deletion, we noted worsening progression of PH in mice without endothelial cell CXCR2. Thus loss of endothelial cell CXCR2 resulted in increased ligand availability, perhaps related to decreased receptor-mediated degradation and induction of a primary vasculopathic extracellular matrix protein, MMP9. Although previously believed to have a primarily deleterious effect in development of pulmonary vasculopathies, endothelial cell CXCR2 expression in our studies is consistent with a protective role against disease.
Importantly, our group has previously demonstrated that pharmacologic blockade of CXCR2 in vivo mimicked the mCXCR2 phenotype, a reduction in RVSP in response to bleomycin-associated injury (8). This observation was associated with the key finding that expansion and stimulation of MDSCs resulted in worsening disease, in both the bleomycin and hypoxia models. However, CXCR2 inhibition appears to be context dependent, based on targeted cell type of expression. Therefore, collectively the present findings offer a cautious reminder in the eventual application of CXCR2 inhibitors to various lung diseases. While multiple classes of drugs have been shown to have promise in preclinical models of pulmonary fibrosis with or without PH, the majority have failed to demonstrate benefit in patients with disease (26, 39) often due to off-target effects. As such, increased understanding of both temporal and localized contributors to disease, such as presence or absence of MDSCs, in distinct preclinical models could identify more efficacious and selective targets of pathological immune cell trafficking and T-cell subtype activation (35). An example would be considering the use of selective CXCR2 inhibitors in combination with an existing drug that inhibits MDSC function, through nonredundant pathways. One such medication, already indicated for PH treatment, is the phosphodiesterase-5 inhibitor sildenafil (34). Intriguingly, to date, this drug is the only well-studied PH medication used in patients with IPF that displays a significant improvement in physiology (as indicated by increased diffusing capacity of the lung for carbon monoxide), quality of life, and dyspnea (21). Finally, one must also consider the effects of concurrent use with existing IPF drugs, such as the tyrosine kinase inhibitor Nintedanib, that may work synergistically in combination with CXCR2 inhibitors (28) or other MDSC active drugs (27). Therefore, further study of the factors mediating vascular changes in patients is needed, specifically the MDSC-mediated mechanism of action in lung remodeling.
Several lines of investigation have previously shown that bone marrow-derived cells are directly involved in extracellular matrix remodeling in PH. For example, experiments with eGFP-bone marrow-transplanted mice exposed to chronic hypoxia demonstrated that PH induces increased bone marrow-derived cell infiltration into the pulmonary parenchyma and is involved in perivascular leukocyte trafficking and subsequent remodeling in the lungs (5). Furthermore, bone marrow-derived proangiogenic cells contribute directly to small-vessel remodeling in PH, and their ablation in the Sugen hypoxia model reduces RVSP, inhibiting the muscularization and stiffening of pulmonary arterial vessels, with no difference in RV remodeling (5). Saliently, there is a noted lack of observed change in right ventricular remodeling correlating to RVSP augmentation in our models. This phenomenon may be due to an improvement in right heart function independent of gross remodeling assessment and would best be evaluated in future studies using functional noninvasive cardiac imaging (16).
In conclusion, we surmised that in those with interstitial lung disease and PH, there is altered CXCR2 axis signaling in both the myeloid and vascular endothelium tissue compartments as compared with those without PH (proposed summary illustration in Fig. 7). These data are consistent with the preclinical animal data, confirming the utility of the animal models in future investigation of disease. Our findings also stress the importance of tissue specificity in consideration of potential therapeutic applications of CXCR2 inhibition and beyond. In particular, our research highlights the need for consideration of off-target effects of existing and upcoming therapies for IPF that may negatively influence MDSC trafficking and selection within the lung, contributing to development of PH. Future studies are therefore required to optimize selected, perhaps combinatorial, agents in treatment of Group 3 PH.
Fig. 7.
Summary figure. Myeloid-derived suppressor cell (MDSC), and not pulmonary microvascular endothelial cell (PMVEC), expression of CXCR2 contributes to aberrant pulmonary vascular remodeling and development of pulmonary hypertension in response to lung injury. MMP9, matrix metallopeptidase 9. PMN, polymorphonuclear.
GRANTS
The authors acknowledge financial support from the National Institutes of Health National Heart, Lung, and Blood Institute Grants K08 HL144085 and R01 HL142776 (A. J. Bryant), Gilead Sciences Research Scholars Program in Pulmonary Arterial Hypertension (A. J. Bryant), Martha Q. Landenberger Research Foundation (A. J. Bryant), American Lung Association (A. J. Bryant), and University of Florida Gatorade Trust (A. J. Bryant).
DISCLOSURES
C. E. Ventetuolo reports personal fees from Acceleron Pharma, personal fees from Bayer, grants to her institution from Eiger and United Therapeutics, and personal fees (travel, editorial work) from American Thoracic Society, outside the submitted work; her spouse is an employee of CVS Health. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.C.O., M.L.B., C.E.V., B.M., E.W.S., and A.J.B. conceived and designed research; A.C.O., C.F., Y.L., M.A.W., L.P., C.E.V., M.K.R., E.W.S., and A.J.B. performed experiments; A.C.O., C.F., Y.L., L.P., and A.J.B. analyzed data; A.C.O., C.F., Y.L., L.P., C.E.V., B.M., and A.J.B. interpreted results of experiments; A.C.O., L.P., and A.J.B. prepared figures; A.C.O., C.E.V., and A.J.B. drafted manuscript; A.C.O., C.F., Y.L., M.A.W., L.P., M.L.B., C.E.V., M.K.R., B.M., E.W.S., and A.J.B. edited and revised manuscript; A.C.O., C.F., Y.L., M.A.W., L.P., M.L.B., C.E.V., M.K.R., B.M., E.W.S., and A.J.B. approved final version of manuscript.
REFERENCES
- 1.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn 235: 759–767, 2006. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 2.Andersen CU, Mellemkjær S, Nielsen-Kudsk JE, Bendstrup E, Simonsen U, Hilberg O. Diagnostic and prognostic role of biomarkers for pulmonary hypertension in interstitial lung disease. Respir Med 106: 1749–1755, 2012. doi: 10.1016/j.rmed.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Babu AN, Murakawa T, Thurman JM, Miller EJ, Henson PM, Zamora MR, Voelkel NF, Nicolls MR. Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis. J Clin Invest 117: 3774–3785, 2007. doi: 10.1172/JCI32311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird GL, Archer-Chicko C, Barr RG, Bluemke DA, Foderaro AE, Fritz JS, Hill NS, Kawut SM, Klinger JR, Lima JAC, Mullin CJ, Ouyang P, Palevsky HI, Palmisicano AJ, Pinder D, Preston IR, Roberts KE, Smith KA, Walsh T, Whittenhall M, Ventetuolo CE. Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease- and congenital heart disease-associated pulmonary arterial hypertension. Eur Respir J 51: 1800467, 2018. doi: 10.1183/13993003.00467-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloodworth NC, Clark CR, West JD, Snider JC, Gaskill C, Shay S, Scott C, Bastarache J, Gladson S, Moore C, D’Amico R, Brittain EL, Tanjore H, Blackwell TS, Majka SM, Merryman WD. Bone marrow-derived proangiogenic cells mediate pulmonary arteriole stiffening via serotonin 2B receptor dependent mechanism. Circ Res 123: e51–e64, 2018. doi: 10.1161/CIRCRESAHA.118.313397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant AJ, Carrick RP, McConaha ME, Jones BR, Shay SD, Moore CS, Blackwell TR, Gladson S, Penner NL, Burman A, Tanjore H, Hemnes AR, Karwandyar AK, Polosukhin VV, Talati MA, Dong HJ, Gleaves LA, Carrier EJ, Gaskill C, Scott EW, Majka SM, Fessel JP, Haase VH, West JD, Blackwell TS, Lawson WE. Endothelial HIF signaling regulates pulmonary fibrosis-associated pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L249–L262, 2016. doi: 10.1152/ajplung.00258.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant AJ, Robinson LJ, Moore CS, Blackwell TR, Gladson S, Penner NL, Burman A, McClellan LJ, Polosukhin VV, Tanjore H, McConaha ME, Gleaves LA, Talati MA, Hemnes AR, Fessel JP, Lawson WE, Blackwell TS, West JD. Expression of mutant bone morphogenetic protein receptor II worsens pulmonary hypertension secondary to pulmonary fibrosis. Pulm Circ 5: 681–690, 2015. doi: 10.1086/683811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant AJ, Shenoy V, Fu C, Marek G, Lorentsen KJ, Herzog EL, Brantly ML, Avram D, Scott EW. Myeloid-derived suppressor cells are necessary for development of pulmonary hypertension. Am J Respir Cell Mol Biol 58: 170–180, 2018. doi: 10.1165/rcmb.2017-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton VJ, Ciuclan LI, Holmes AM, Rodman DM, Walker C, Budd DC. Bone morphogenetic protein receptor II regulates pulmonary artery endothelial cell barrier function. Blood 117: 333–341, 2011. doi: 10.1182/blood-2010-05-285973. [DOI] [PubMed] [Google Scholar]
- 10.Burton VJ, Holmes AM, Ciuclan LI, Robinson A, Roger JS, Jarai G, Pearce AC, Budd DC. Attenuation of leukocyte recruitment via CXCR1/2 inhibition stops the progression of PAH in mice with genetic ablation of endothelial BMPR-II. Blood 118: 4750–4758, 2011. doi: 10.1182/blood-2011-05-347393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi YH, Burdick MD, Strieter BA, Mehrad B, Strieter RM. CXCR4, but not CXCR7, discriminates metastatic behavior in non-small cell lung cancer cells. Mol Cancer Res 12: 38–47, 2014. doi: 10.1158/1541-7786.MCR-12-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cottin V, Price LC, Valenzuela C. The unmet medical need of pulmonary hypertension in idiopathic pulmonary fibrosis. Eur Respir J 51: 1702596, 2018. doi: 10.1183/13993003.02596-2017. [DOI] [PubMed] [Google Scholar]
- 13.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 53: 585–600, 2015. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Däbritz J, Judd LM, Chalinor HV, Menheniott TR, Giraud AS. Altered gp130 signalling ameliorates experimental colitis via myeloid cell-specific STAT3 activation and myeloid-derived suppressor cells. Sci Rep 6: 20584, 2016. doi: 10.1038/srep20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez IE, Greiffo FR, Frankenberger M, Bandres J, Heinzelmann K, Neurohr C, Hatz R, Hartl D, Behr J, Eickelberg O. Peripheral blood myeloid-derived suppressor cells reflect disease status in idiopathic pulmonary fibrosis. Eur Respir J 48: 1171–1183, 2016. doi: 10.1183/13993003.01826-2015. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, Taraseviciene-Stewart L, Sung Y, Kraskauskas D, Farkas D, Conrad DH, Nicolls MR, Voelkel NF. A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects. Am J Physiol Lung Cell Mol Physiol 302: L977–L991, 2012. doi: 10.1152/ajplung.00362.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, Maynard KB, Gleaves L, Talati M, Absi T, Disalvo T, West J. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 189: 325–334, 2014. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med 6: 237ra67, 2014. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogmalm A, Bäckström E, Bry M, Lappalainen U, Lukkarinen HP, Bry K. Role of CXC chemokine receptor-2 in a murine model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 47: 746–758, 2012. doi: 10.1165/rcmb.2011-0394OC. [DOI] [PubMed] [Google Scholar]
- 20.Hou Y, Wu Y, Farooq SM, Guan X, Wang S, Liu Y, Oblak JJ, Holcomb J, Jiang Y, Strieter RM, Lasley RD, Arbab AS, Sun F, Li C, Yang Z. A critical role of CXCR2 PDZ-mediated interactions in endothelial progenitor cell homing and angiogenesis. Stem Cell Res (Amst) 14: 133–143, 2015. doi: 10.1016/j.scr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Idiopathic Pulmonary Fibrosis Clinical Research Network, Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med 363: 620–628, 2010. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, Ager C, Nicholas C, Jaiswal AR, Sun Y, Shah K, Balasubramanyam S, Li N, Wang G, Ning J, Zal A, Zal T, Curran MA. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest 128: 5137–5149, 2018. doi: 10.1172/JCI96268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Judge EP, Fabre A, Adamali HI, Egan JJ. Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J 40: 93–100, 2012. doi: 10.1183/09031936.00115511. [DOI] [PubMed] [Google Scholar]
- 24.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 24: 631–644, 2013. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keane MP, Arenberg DA, Lynch JP 3rd, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, Kunkel SL, Strieter RM. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol 159: 1437–1443, 1997. [PubMed] [Google Scholar]
- 26.King TE Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, Valeyre D, Leconte I, Morganti A, Roux S, Behr J. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 184: 92–99, 2011. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 27.Kolb M, Raghu G, Wells AU, Behr J, Richeldi L, Schinzel B, Quaresma M, Stowasser S, Martinez FJ; INSTAGE Investigators . Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med 379: 1722–1731, 2018. doi: 10.1056/NEJMoa1811737. [DOI] [PubMed] [Google Scholar]
- 28.Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P, Behera R, Goins MA, Mulligan C, Nam B, Hockstein N, Denstman F, Shakamuri S, Speicher DW, Weeraratna AT, Chao T, Vonderheide RH, Languino LR, Ordentlich P, Liu Q, Xu X, Lo A, Puré E, Zhang C, Loboda A, Sepulveda MA, Snyder LA, Gabrilovich DI. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 32: 654–668.e5, 2017. doi: 10.1016/j.ccell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, Li B, Donnelly EF, Holburn GE, Lewis KG, Collins RD, Hull WM, Glasser SW, Whitsett JA, Blackwell TS. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol 167: 1267–1277, 2005. doi: 10.1016/S0002-9440(10)61214-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesina M, Wörmann SM, Morton J, Diakopoulos KN, Korneeva O, Wimmer M, Einwächter H, Sperveslage J, Demir IE, Kehl T, Saur D, Sipos B, Heikenwälder M, Steiner JM, Wang TC, Sansom OJ, Schmid RM, Algül H. RelA regulates CXCL1/CXCR2-dependent oncogene-induced senescence in murine Kras-driven pancreatic carcinogenesis. J Clin Invest 126: 2919–2932, 2016. doi: 10.1172/JCI86477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 129: 746–752, 2006. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 32.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170: 3369–3376, 2003. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 33.Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis 8: 63–71, 2005. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 34.Lin S, Wang J, Wang L, Wen J, Guo Y, Qiao W, Zhou J, Xu G, Zhi F. Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC. Am J Cancer Res 7: 41–52, 2017. [PMC free article] [PubMed] [Google Scholar]
- 35.Maston LD, Jones DT, Giermakowska W, Howard TA, Cannon JL, Wang W, Wei Y, Xuan W, Resta TC, Gonzalez Bosc LV. Central role of T helper 17 cells in chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 312: L609–L624, 2017. doi: 10.1152/ajplung.00531.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res 67: 11438–11446, 2007. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyake M, Goodison S, Urquidi V, Gomes Giacoia E, Rosser CJ. Expression of CXCL1 in human endothelial cells induces angiogenesis through the CXCR2 receptor and the ERK1/2 and EGF pathways. Lab Invest 93: 768–778, 2013. doi: 10.1038/labinvest.2013.71. [DOI] [PubMed] [Google Scholar]
- 38.Pi L, Fu C, Lu Y, Zhou J, Jorgensen M, Shenoy V, Lipson KE, Scott EW, Bryant AJ. Vascular endothelial cell-specific connective tissue growth factor (CTGF) is necessary for development of chronic hypoxia-induced pulmonary hypertension. Front Physiol 9: 138, 2018. doi: 10.3389/fphys.2018.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, Martinez FJ, Nathan SD, Wells AU, Collard HR, Costabel U, Richeldi L, de Andrade J, Khalil N, Morrison LD, Lederer DJ, Shao L, Li X, Pedersen PS, Montgomery AB, Chien JW, O’Riordan TG; ARTEMIS-IPF Investigators* . Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med 158: 641–649, 2013. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 40.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest 116: 695–702, 2006. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribechini E, Leenen PJ, Lutz MB. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur J Immunol 39: 3538–3551, 2009. doi: 10.1002/eji.200939530. [DOI] [PubMed] [Google Scholar]
- 43.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, Klesen M, Zhang Y, Gibson KF. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 185: 67–76, 2012. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivera-Lebron BN, Forfia PR, Kreider M, Lee JC, Holmes JH, Kawut SM. Echocardiographic and hemodynamic predictors of mortality in idiopathic pulmonary fibrosis. Chest 144: 564–570, 2013. doi: 10.1378/chest.12-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo RC, Guabiraba R, Garcia CC, Barcelos LS, Roffê E, Souza AL, Amaral FA, Cisalpino D, Cassali GD, Doni A, Bertini R, Teixeira MM. Role of the chemokine receptor CXCR2 in bleomycin-induced pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol 40: 410–421, 2009. doi: 10.1165/rcmb.2007-0364OC. [DOI] [PubMed] [Google Scholar]
- 46.Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, Eberlein C, Candido JB, Clarke M, Nixon C, Connelly J, Jamieson N, Carter CR, Balkwill F, Chang DK, Evans TRJ, Strathdee D, Biankin AV, Nibbs RJB, Barry ST, Sansom OJ, Morton JP. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 29: 832–845, 2016. doi: 10.1016/j.ccell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su X, Yang L, Yin Y, Huang J, Qiao F, Fang Y, Yu L, Wang Y, Zhou K, Wang J. Bone marrow mesenchymal stem cells tune the differentiation of myeloid-derived suppressor cells in bleomycin-induced lung injury. Stem Cell Res Ther 9: 253, 2018. doi: 10.1186/s13287-018-0983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, Klinger JR, Lima JA, Ouyang P, Palevsky HI, Palmisciano AJ, Krishnan I, Pinder D, Preston IR, Roberts KE, Kawut SM. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med 193: 1168–1175, 2016. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, Zhao K, Konaparthi R, Hua S, Zhang J, Li-Ning-Tapia EM, Kapoor A, Wu CJ, Patel NB, Guo Z, Ramamoorthy V, Tieu TN, Heffernan T, Zhao D, Shang X, Khadka S, Hou P, Hu B, Jin EJ, Yao W, Pan X, Ding Z, Shi Y, Li L, Chang Q, Troncoso P, Logothetis CJ, McArthur MJ, Chin L, Wang YA, DePinho RA. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov 6: 80–95, 2016. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, Jiao Z, Duan T, Liu M, Zhu B, Zhang Y, Xu Q, Wang R, Xiong Y, Xu H, Lu L. Functional characterization of myeloid-derived suppressor cell subpopulations during the development of experimental arthritis. Eur J Immunol 45: 464–473, 2015. doi: 10.1002/eji.201444799. [DOI] [PubMed] [Google Scholar]
- 51.Wang XM, Shi K, Li JJ, Chen TT, Guo YH, Liu YL, Yang YF, Yang S. Effects of angiotensin II intervention on MMP-2, MMP-9, TIMP-1, and collagen expression in rats with pulmonary hypertension. Genet Mol Res 14: 1707–1717, 2015. doi: 10.4238/2015.March.6.17. [DOI] [PubMed] [Google Scholar]
- 52.Yeager ME, Nguyen CM, Belchenko DD, Colvin KL, Takatsuki S, Ivy DD, Stenmark KR. Circulating myeloid-derived suppressor cells are increased and activated in pulmonary hypertension. Chest 141: 944–952, 2012. doi: 10.1378/chest.11-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziegenhagen MW, Zabel P, Zissel G, Schlaak M, Müller-Quernheim J. Serum level of interleukin 8 is elevated in idiopathic pulmonary fibrosis and indicates disease activity. Am J Respir Crit Care Med 157: 762–768, 1998. doi: 10.1164/ajrccm.157.3.9705014. [DOI] [PubMed] [Google Scholar]