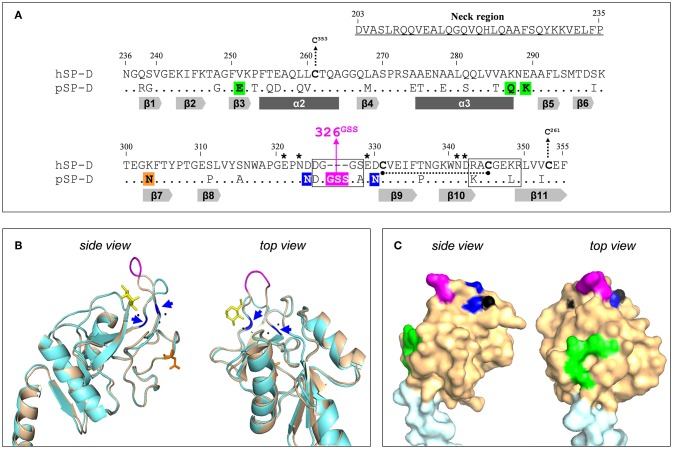
Figure 1.
Design of the NCRD of iSP-D. (A) Amino acid sequence alignment of the CRD sequences from human SP-D (hSP-D, Genbank accession code: X65018) and porcine SP-D (pSP-D, AF132496). Residue numbering according to hSP-D sequence. Dashes indicate spaces that are inserted to maximize the identity across the alignment. The residues that comprise the neck region of hSP-D are underlined (residues #203-#235). Dots indicate identical residues between hSP-D and pSP-D. Dashes indicate spaces inserted to maximize the identity across the alignment. Mutated residues in hSP-D that generate iSP-D are shaded in magenta, blue and green. The N-glycosylation site at #303 in the CRD of pSP-D is indicated (orange box). The secondary structural regions are indicated as gray arrows (β-sheets) and dark gray bars (α-helices), deduced from the crystal structure of rat SP-A (18). SP-D groove regions (delineated), cysteines (bold) and cysteine bridges (black dotted lines/arrows), and key residues necessary for coordination of Ca2+-ions and hydroxyl groups of oligosaccharides (asterisks) are also indicated. (B) Tertiary protein structure of the neckCRD of SP-D. Shown is a ribbon diagram of the NCRD of hSP-D (blue, PDB accession no. 3G81) overlayed with that of pSP-D (brown, PDB accession no. 4DN8). A bound α-methyl mannoside as stick representation in yellow is visualized to illustrate the lectin binding pocket and 3 calcium ions are shown as black spheres. Modifications of hSP-D present in iSP-D are highlighted with deviating colors: the 326GSS-insertion (magenta), and the Asp324Asn and Asp330Asn mutations (blue; also indicated with blue arrows). Location of the N-linked glycan in the CRD of pSP-D is shown in orange sticks. (C) Surface representation of the neckCRD of pSP-D. Shown is the neck region (light blue) and the CRD (brown) of pSP-D (PDB accession no 4DN8) with porcine-specific residues mutated into hSP-D highlighted in different colors: the 326GSS-insertion (magenta), and the Man8-contact residues Glu251, Gln287, and Lys289 (green). Calcium ions are only partly visible on the surface (black).