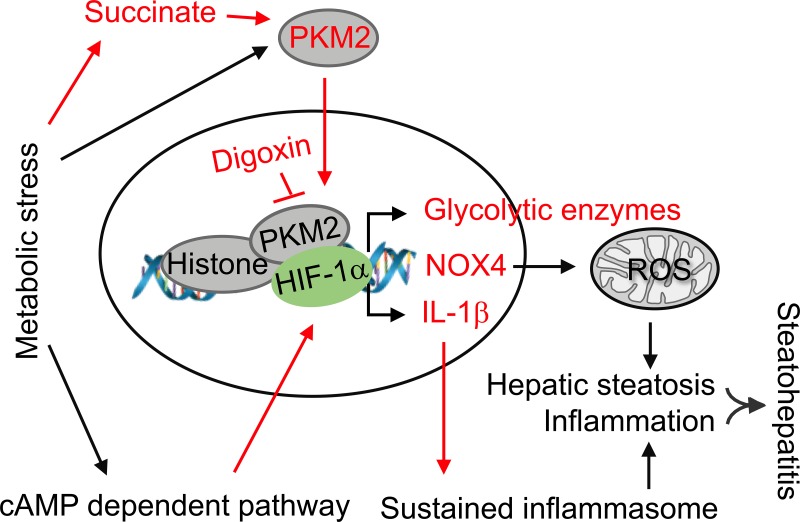
Fig. 8.
Working model of digoxin inhibited liver injury in nonalcoholic steatohepatitis (NASH). The cell metabolic stress generated from high-fat diet (HFD) intake in the mouse triggers pyruvate kinase M2 (PKM2) activation that can occur partially via metabolites like succinate. In addition to its pyruvate kinase function, PKM2 interacts with hypoxia-inducible factor-1α (HIF-1α) in the nucleus and functions as a transcriptional coactivator for HIF-1α, resulting in stimulation of HIF-1α-responsive genes. We have previously shown that chronic metabolic stress triggers cAMP pathway activation, which results in HIF-1α transactivation leading sustained IL-1β gene expression and inflammasome activation, and digoxin reduces this pathway-driven sustained inflammation. Thus, digoxin has the ability to improve nonalcoholic fatty liver disease (NAFLD)/nonalcoholic fatty liver disease (NASH) through the inhibition of PKM2-HIF1α pathway that targets both oxidative stress and proinflammatory gene expression. Labels and arrows highlighted in red indicate the new findings from our work.