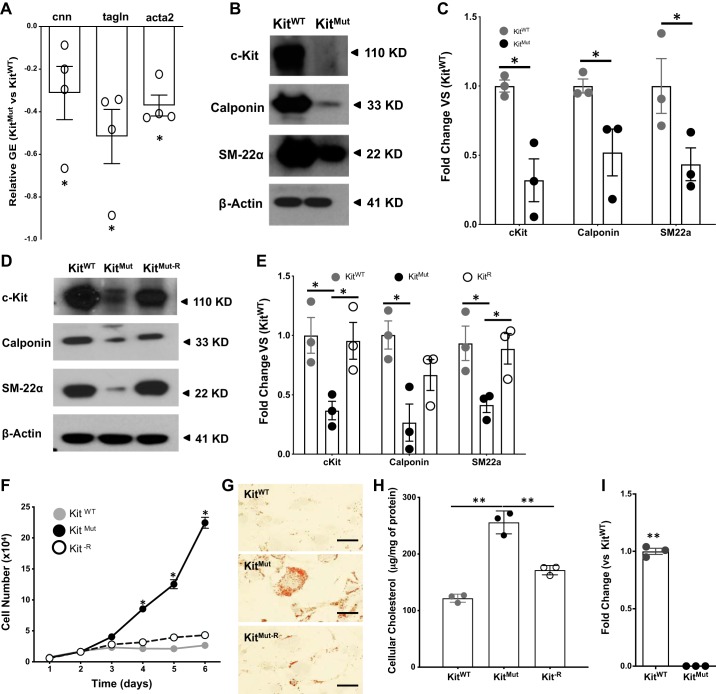
Fig. 5.
c-Kit deficiency facilitates the synthetic phenotype in smooth muscle cells (SMCs) of c-Kit mutant mice. A–C: downregulation of contractile gene expression (GE) in aortas of KitMut versus KitWT, as determined by quantitative RT-PCR (A) and Western blot (B and C). Protein expression in C was normalized using β-actin levels and then standardized against KitWT. The P value was assessed using a 2-tailed t-test assuming unequal variance. D and E: reconstitution of c-Kit restored the expression of contractile markers in cultured SMCs, as determined by Western blot. Proteins were extracted from primary KitWT SMCs and primary KitMut SMCs transduced with either a lentiviral vector containing the murine c-Kit cDNA under a PGK promoter (KitMut-R) or the empty lentivirus (KitMut). Data are presented as means ± SE. Protein expression was normalized using β-actin levels and then standardized against KitWT. Groups were compared using a 1-way ANOVA, followed by a Newman-Keuls test. F–H: reconstitution of c-Kit (KitMut-R) slowed down proliferation of c-Kit mutant SMCs (F) and prevented intracellular accumulation of cholesterol as detected with Oil Red O staining (G). Cellular cholesterol levels were determined after 72 h culture in Chol:MβCD-loaded (20 μg/ml) 0.2% BSA medium and standardized against whole lysate protein concentration (H). Scale bars, 40 µm. Data in the bar graph are presented as means ± SE. The P values were assessed using a 1-way ANOVA, followed by a Newman-Keuls test. I: deficient c-Kit activity led to significant downregulation of the ATP-binding cassette subfamily G member 1 gene (ABCG1). Data in the bar graph are presented as means ± SE. The P value was assessed using a 2-tailed t-test assuming equal variance. *P < 0.05; **P < 0.01.