Abstract
Ischemic heart diseases such as myocardial infarction (MI) are the largest contributors to cardiovascular disease worldwide. The resulting cardiac cell death impairs function of the heart and can lead to heart failure and death. Reperfusion of the ischemic tissue is necessary but causes damage to the surrounding tissue by reperfusion injury. Cortical bone stem cells (CBSCs) have been shown to increase pump function and decrease scar size in a large animal swine model of MI. To investigate the potential mechanism for these changes, we hypothesized that CBSCs were altering cardiac cell death after reperfusion. To test this, we performed TUNEL staining for apoptosis and antibody-based immunohistochemistry on tissue from Göttingen miniswine that underwent 90 min of lateral anterior descending coronary artery ischemia followed by 3 or 7 days of reperfusion to assess changes in cardiomyocyte and noncardiomyocyte cell death. Our findings indicate that although myocyte apoptosis is present 3 days after ischemia and is lower in CBSC-treated animals, myocyte apoptosis accounts for <2% of all apoptosis in the reperfused heart. In addition, nonmyocyte apoptosis trends toward decreased in CBSC-treated hearts, and although CBSCs increase macrophage and T-cell populations in the infarct region, the occurrence of apoptosis in CD45+ cells in the myocardium is not different between groups. From these data, we conclude that CBSCs may be influencing cardiomyocyte and noncardiomyocyte cell death and immune cell recruitment dynamics in the heart after MI, and these changes may account for some of the beneficial effects conferred by CBSC treatment.
NEW & NOTEWORTHY The following research explores aspects of cell death and inflammation that have not been previously studied in a large animal model. In addition, apoptosis and cell death have not been studied in the context of cell therapy and myocardial infarction. In this article, we describe interactions between cell therapy and inflammation and the potential implications for cardiac wound healing.
Listen to this article's corresponding podcast at https://ajpheart.podbean.com/e/cortical-bone-derived-stem-cell-therapy-reduces-apoptosis/.
Keywords: apoptosis, ischemia, myocardial infarction, reperfusion, stem cell therapy
INTRODUCTION
Cardiovascular diseases account for the largest proportion of premature mortality and years lived with disability of any noncommunicable disease (23). Ischemic heart disease, such as acute myocardial infarction (AMI), is the largest contributor to the epidemic of cardiovascular disease. AMI occurs when the coronary arteries are blocked, preventing adequate perfusion of downstream myocardium (2). Prolonged ischemia can lead to irreversible cell death that decreases global function of the heart. Over time, the damaged myocardium is replaced with noncontractile scar, contributing to left ventricle (LV) dysfunction and often leading to the development of heart failure with reduced ejection fraction, a major cause of morbidity and mortality after AMI (7).
The standard current therapy for AMI is timely reperfusion of the infarcted tissue by primary percutaneous intervention. Clearance of the blocked artery salvages ischemic myocardium, restores electrical stability preventing deadly arrhythmias, and improves short- and long-term outcomes for AMI patients. However, the reperfusion of damaged tissue can also exacerbate infarct size and cardiomyocyte cell death through what is now termed reperfusion injury (RI) (25). Animal studies have shown that RI can account for up to 50% of infarct size after AMI, highlighting the importance of treating this aspect of cardiac damage (33).
Beyond the immediate changes to cells in the ischemic zone, reperfusion of the myocardium causes changes throughout the body that can last days or weeks after AMI. Initially, the extreme physiological conditions of ischemia cause cardiac cells to undergo necrosis primarily, the rapid and uncontrolled cellular death and release of intracellular contents into the intercellular space. Necrosis begins rapidly after the onset of AMI and lasts up to 24 h (26). Concurrently, the caspase-dependent programmed cell death, termed apoptosis, occurs within minutes of AMI and can last days to weeks in humans, resulting in further cardiomyocyte loss after reperfusion (1, 32). The death of cardiomyocytes and other cardiac cells after AMI is the proximal cause of cardiac dysfunction and initiates wound healing that involves inflammation, resolution, and scar formation. Therapies specifically targeting these aspects of RI-induced cell death include measures to activate prosurvival pathways, deactivate the pathways involved in cell death, and improve metabolism in ischemic tissue (25).
These treatments are primarily focused on preventing cardiomyocyte damage and loss to preserve the overall function of the heart. However, in addition to cardiomyocyte death, RI activates and induces the recruitment of numerous other cell types that are inextricably involved in infarct healing and remodeling. Most notable are the litany of immune cells that rapidly infiltrate the infarcted myocardium, remain in the tissue up to months after AMI, and are absolutely required for proper infarct healing (12, 13, 19, 20). These immune cells play vital roles in the clearance of dead cells and the formation of scar. The death and clearance of immune cells themselves is a major factor in wound healing after AMI. RI and its therapeutic targets have been summarized frequently in recent years, and there are a number of known mechanisms through which RI further damages the myocardium. Despite the knowledge of how and why RI occurs, translational therapies directed toward the mitigation of RI have been largely unsuccessful (5, 18, 25, 33).
Cell therapies aimed at treating the injured or failing heart after AMI are typically not included in the discussion of RI and potential targets of treatment. However, the immunomodulatory, prohealing properties of many stem cell types make them intriguing candidates for the prevention of cell death due to RI. Furthermore, various stem cell types have already undergone clinical trials, and although improvements in cardiac function in these studies are modest, allogeneic stem cell therapy has been shown to be safe for human patients (17). Our group has characterized a stem cell population isolated from the cortex of bone [cortical bone stem cells (CBSCs)] that are distinct from mesenchymal and cardiac-derived stem cells and are cardioprotective both in vitro and in vivo, significantly reducing scar size and improving ventricular function in both small and large animal models (11, 22, 28). CBSCs show unique expression of immunomodulatory proteins and cytokine production that may account for these most striking effects. Recently, we tested these cells in a translational preclinical large animal model of AMI using Göttingen miniswine and found that in addition to improved parameters of LV function at 3 mo, there was a decrease in terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive apoptotic nonmyocytes in CBSC-treated animals 3 days after AMI, as well as fewer TUNEL-positive myocytes and nonmyocytes 3 mo after AMI (28).
On the basis of the decrease in nonmyocyte apoptosis, we hypothesize that CBSCs decrease apoptosis in infiltrating immune cells activated by RI during and after the primary inflammatory response. To test this hypothesis, heart tissue from pigs that received an AMI by balloon blockage of the lateral anterior descending coronary artery (LAD), intramyocardial injections of saline (vehicle) or CBSCs, and 3 or 7 days of reperfusion as previously described (28) was fixed, embedded, slide-mounted, and stained for TUNEL-positive apoptotic cells, CD45 hematopoietic-derived blood cells, T cells, and macrophages to assess the extent of apoptosis in the ischemic heart.
METHODS
Animals, anesthesia induction, and maintenance.
All surgical procedures in this study were reviewed and approved by the Temple University Institutional Animal Care and Use Committee (IACUC). All information pertaining to the animals receiving 3 days of reperfusion after AMI has been published previously (28). The heart tissue analyzed in the 3-day group was sourced from the animals included in that study (CBSCs n = 4, vehicle n = 4). The animals in the 7-day group received the same surgery with minor modifications. These animals were part of a larger study assessing the immunomodulatory aspects of CBSC treatment. Only the relevant methods and procedures for the data described in this paper are listed. Briefly, 14 female Göttingen miniswine were purchased from Marshall BioResources (North Rose, NY). All animals were ages 9–12 mo and weighed between 25 and 30 kg at the time of surgery. Fourteen animals underwent surgery to receive an AMI. Anesthesia was induced first by intramuscular injection of 6.0 mg/kg tiletamine-zolazapam (Telazol; Fort Dodge Animal Health, Fort Dodge, IA). Animals were then intubated with a 5.5-mm internal diameter endotracheal tube, and general anesthesia was maintained with 1.5–2% isoflurane (IsoFlo; Zoetis, Kalamazoo, MI).
Isolation of CBSCs.
CBSCs were isolated as previously described (28). Briefly, CBSCs were isolated from a male Göttingen miniswine that was sedated, intubated, and anesthetized as described above. Under sterile conditions, an incision was made in the right hind limb to access the tibia. An Osteo-Site bone biopsy needle (Cook Medical, Bloomington, IN) was used to collect a transmural bone biopsy from the periosteum to medullar cavity. The biopsy was put through a series of digestions in 0.25% collagenase type I (StemCell Technologies, Vancouver, Canada) and filtered to remove any debris. Cells were passaged and characterized as previously described (11, 22). Once characterized, the cells were infected with green fluorescent protein (GFP) lentivirus as previously described and sorted on a BD FACS Aria flow cytometry machine to ensure a >99% GFP+ population. The cells were expanded and, once enough cells were generated, removed from the tissue-culture plates and resuspended in sterile phosphate-buffered saline (PBS) at a concentration of 4 × 106 CBSCs/mL. Then 0.5 mL of resuspended cells were drawn into 1-mL sterile syringes and kept on ice until injection.
Myocardial infarction-induced ischemia-reperfusion injury.
Myocardial infarction was induced by percutaneous transluminal coronary angioplasty as previously described (28, 31). An angioplasty balloon was guided through the femoral artery to the mid-LAD past the first diagonal branch. The balloon was inflated in the LAD for 90 min. Location and ischemia were confirmed by perfusion of the coronary arteries with the radiopaque contrast iopamidol (ISOVUE; Bracco Diagnostic, Milan, Italy) and fluoroscopy. Three animals developed cardiac arrhythmias at the time of infarction and could not be resuscitated. These animals were euthanized immediately following confirmation of pulseless electrical activity by electrocardiogram and akinesis of the heart by fluoroscopy as per Temple University IACUC protocol. Eleven animals (78% survival rate, consistent with previous studies) survived the AMI. One animal did not meet the requirements for injection site recovery (>70%) and was excluded from the study (vehicle n = 4, CBSC n = 6).
Preparation and intramyocardial injection of CBSCs.
CBSCs were injected directly into the myocardium using the NOGA mapping and injection system as previously described (28). Briefly, the interior of the heart was mapped based on the electrical conductance of the tissue using a NOGA mapping catheter. This map was used to determine the border zone (BZ) of the infarct (infarcted tissue conducts electricity differently from healthy tissue), and an injection catheter was then inserted. Ten 0.5-mL injections of 2 million cells each (2 × 107 cells total) and fluorescent FluoSpheres were injected directly into the myocardium of the BZ 1.5–2 h after MI, which helped to identify the injection sites.
Tissue collection and processing.
On the day of terminal surgery (days 3 or 7), animals were euthanized by removal of the heart under deep anesthesia. The heart was flushed 3 times by the injection of 60 mL sterile saline solution into the aorta. The heart was then cut in 8–10-mm slices from apex to base, and select regions were removed by 3-mm punch biopsy and snap-frozen in liquid nitrogen. Injection sites were located using fluorescent polystyrene FluoSpheres. These beads are mixed with the cells before injection and glow under ultraviolet light. They remain in the tissue regardless of cell presence at least 3 mo post-MI. The rest of the heart was fixed in Buffered Formalde-Fresh low-odor 10% formalin (Fisher Chemical, Fair Lawn, NJ). After fixation for at least 72 h, the hearts were sectioned by zone based on gross anatomical analysis of tissue damage and scar development. The sections were kept in formalin and processed using a Leica ASP300S (Leica Microsystems, Bannockburn, IL). The slices were then embedded in Histoplast LP paraffin wax (Thermo Scientific, Waltham, MA), sliced in 5-µm sections, and mounted on glass slides by AML (Jacksonville, FL).
Blinding and treatment assignment.
Before beginning the study, each animal was given a four-digit numerical code pertaining to their assigned United States Department of Agriculture number. On the day of AMI surgery, each animal was randomly assigned to either the vehicle treatment arm or the CBSC treatment arm by an individual not participating in any other aspect of the study. The treatment of each animal was unknown to every researcher involved in the surgery, data collection, or analysis for the duration of the study. Once the data were fully analyzed, the treatment of each animal was revealed to compare groups.
TUNEL and immunofluorescence staining.
TUNEL staining was performed with the DeadEnd Fluorometric TUNEL System kit (Promega, Madison, WI). The protocol was followed exactly. Briefly, slides were deparaffinized by submersion in xylene (Fisher Scientific, Fair Lawn, NJ) and rehydrated in decreasing concentrations of ethanol (100, 90, 85, 70, and 50%, sequentially) (Fisher Scientific, Fair Lawn, NJ). The slides were then fixed in 4% paraformaldehyde solution in PBS (Affymetrix, Cleveland, OH). After washing in PBS, the tissue was digested by incubation with the Proteinase K included in the Promega kit for 8 min as per protocol instructions. Slides were then washed and incubated with the labeling cocktail for 1 h at 37°C. The reaction was then stopped with the included SSC solution, and the slides were washed. The slides were fixed once more with paraformaldehyde before immunohistochemical staining, the only divergence from the protocol.
After the final fixation, the slides underwent antigen retrieval, blocking, and staining for CD45 and α-sarcomeric actinin. Briefly, the slides were washed and submerged in 10 mM anhydrous citrate solution, pH 6 (Sigma-Aldrich, St. Louis, MO), then microwaved at 100% power for 2 min and 50% power for 6 min for antigen retrieval. After the slides cooled, they were washed in PBS and blocked using the TNB blocking solution from the TSA Plus Fluorescence kit (PerkinElmer, Waltham, MA). After blocking, the slides were incubated with primary antibodies overnight at 4°C. The next day, the slides were washed in PBS and then incubated with secondary antibodies directed toward the primary antibodies {donkey anti-rabbit IgG Rhodamine [tetramethylrhodamine isothiocyanate (TRITC)] (Jackson ImmunoResearch, West Grove, PA); donkey anti-mouse IgM Cy-5 (Jackson ImmunoResearch)} for 1 h at room temperature. Slides were then washed and incubated with 1 µg/mL 4′,6-diamidino-2-phenylindole (DAPI) (MilliporeSigma, Burlington, MA) for 10 min at room temperature. They were then coverslipped using Vectashield HardSet (Vector, Burlingame, CA) and imaged on a confocal microscope (described below). Staining for CD4 and Mac-2 were done independently of TUNEL staining. The antigen retrieval step consisted of submersion in the same anhydrous citrate solution, but the slides were microwaved for 3 min at 100% power and 12 min at 50% power. Blocking and antibody incubation were performed as described previously. The same secondary, donkey anti-rabbit IgG Rhodamine (TRITC) (Jackson ImmunoResearch) was used to visualize both antibodies on independent slides. Coverslipping was performed as described above. The antibodies used in this study are listed below (Table 1).
Table 1.
Antibodies employed in this study
| Marker | Company | Catalog No. | Dilution |
|---|---|---|---|
| CD45 | Abcam | Ab-10558 | 1 μg/mL |
| α-Sarcomeric actinin | Sigma-Aldrich | A-2172 | 1:100 (ascites fluid in sodium azide) |
| Mac-2 | CedarLane | CL-8942-AP | 10 μg/mL |
| CD4 | Abcam | Ab-133616 | 1.66 μg/mL |
| DAPI | MilliporeSigma | 268298 | 1 μg/mL |
Confirmation of CBSC presence in the tissue.
A GFP-labeling method was employed to confirm the presence of CBSCs in the tissue as previously described (28). Briefly, the CBSCs injected were infected with GFP lentivirus as in previous studies. By identifying regions of injection by the fluorescent beads injected in tandem with the CBSCs, we were able to target GFP staining to only regions of the tissue that received an injection. GFP staining was done using the same immunofluorescence protocol as described in the previous section. Slides were incubated with unconjugated chicken IgY anti-GFP primary (cat. no. A-10262; Invitrogen) to amplify the GFP signal and subsequently incubated with conjugated donkey anti-chicken FITC antibody (cat. no. 703-095-155; Jackson ImmunoResearch).
Image acquisition and analysis.
For all slides, images were taken on a Nikon Eclipse Ti confocal microscope (Nikon, Tokyo, Japan) with a ×20 objective. Representative images were taken with the same ×20 objective with ×2 optical zoom to enhance detail. Images were analyzed using the NIS Elements imaging software. Briefly, each channel (FITC, TRITC, and DAPI) was restricted to exclude background and points of autofluorescence as well as nuclei less than 4 µm2. α-Sarcomeric actinin was used to determine if green TUNEL+ nuclei were within myocytes. Once restricted, each color was turned into a binary layer, and binary layers could be overlapped to assess only regions that were present in both layers. This method was used to determine the colocalization of TUNEL and DAPI, as well as the colocalization of TUNEL, DAPI, and the antibodies used. The Object Count function was used to determine the number these cells as well as the total number of nuclei and total area analyzed.
Mouse proteome profiler cytokine array.
To assess a multitude of cytokines within cell-culture media simultaneously, the Proteome Profiler Mouse Cytokine Array Kit, Panel A (R&D Systems cat. no. ARY-006, Minneapolis, MN) was used. Briefly, CBSCs were isolated and cultured in CBSC media as previously described (11). To generate “conditioned media,” 250,000 CBSCs were cultured in 1 mL of CBSC media for 48 h on sterile Nunclon Delta Surface 6-well plates (cat. no. 140675; ThermoScientific). The media was then removed from the cells and spun at 400 × g to remove any debris and any nonadhered cells. The media was then used in the Proteome Profiler kit as directed by the protocol. The blots were read using a Li-Cor Odyssey Crx (Li-Cor Biosciences, Lincoln, NE), and densitometry was performed with Image Studio analysis software provided by Li-Cor.
Isolation and culture of bone marrow-derived macrophages.
Bone marrow was isolated from a single female C57BL/6J mouse (cat. no. 000664; Jackson, Bar Harbor, ME). Briefly, the mouse was anesthetized with 5% isoflurane and euthanized by cervical dislocation as approved by the Temple University IACUC. The legs were then rapidly removed with the femur and tibia intact and placed in sterile PBS. Muscle tissue was removed, and the head of the femur and tibia were removed once free of muscle tissue. The femur and tibia were flushed with sterile PBS, and the bone marrow was filtered through 100-µm and 40-µm filters subsequently to remove any clumps of cells. Red blood cells were lysed with lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 100 µM EDTA, pH 7.3 in 1 liter of double-distilled H2O filtered through a 0.22-µm filter to sterilize), and the cells were plated on sterile Nunclon Delta Surface 6-well plates as previously described at a concentration of 500,000 cells/well in 2 mL of RPMI 1640 + GlutaMAX-I (cat. no. 72400-047; Gibco, Gaithersburg, MD) with 10% heat inactivated fetal bovine serum (cat. no. 10082139; ThermoFisher) and Pen-strep-glutamine (cat. no. 10378016; Gibco). The media was supplemented with 50 ng/mL M-CSF (Peprotech cat. no. 315-02, Rocky Hill, NJ) to induce a macrophage phenotype in the bone marrow cells. The cells were cultured for 7 days, with removal and replenishment of 1 mL of media every other day. On the seventh day, 1 mL of conditioned or unconditioned CBSC media was added to the macrophages after removal of all RPMI media. The macrophages were incubated in this media for 24 h, and then the media was removed, centrifuged, and used in the Proteome Profiler as previously described.
Quantitative proteomics of frozen tissue.
Tissue punch biopsies of the myocardium infarct zone (IZ), BZ, and remote zone were frozen in liquid nitrogen and stored at −80°C. They were then sent on dry ice to RayBiotech for their Quantibody Multiplex ELISA assays for IL-1β, interleukin-1 receptor antagonist (IL-1RA), chemokine C-C motif ligand 2 (CCL2), and tissue inhibitor of metalloproteinase metallopeptidase inhibitor 1 (TIMP-1).
Statistical analysis.
All comparisons between CBSCs and vehicle as averages were performed by the two-tailed unpaired t test for significance. If the standard deviation was found to be significantly different between groups, a two-tailed unpaired t test with Welch’s correction was applied to the data to correct for the differences in standard deviation. All comparisons between the IZ and BZ of both vehicle and CBSC groups were performed by a two-way ANOVA with Sidak’s method to correct for multiple comparisons. Statistical analysis was performed with GraphPad Prism software (GraphPad Software, San Diego, CA).
RESULTS
CBSC treatment alters cardiomyocyte apoptosis after 3 days of reperfusion and noncardiomyocyte apoptosis after 7 days of reperfusion.
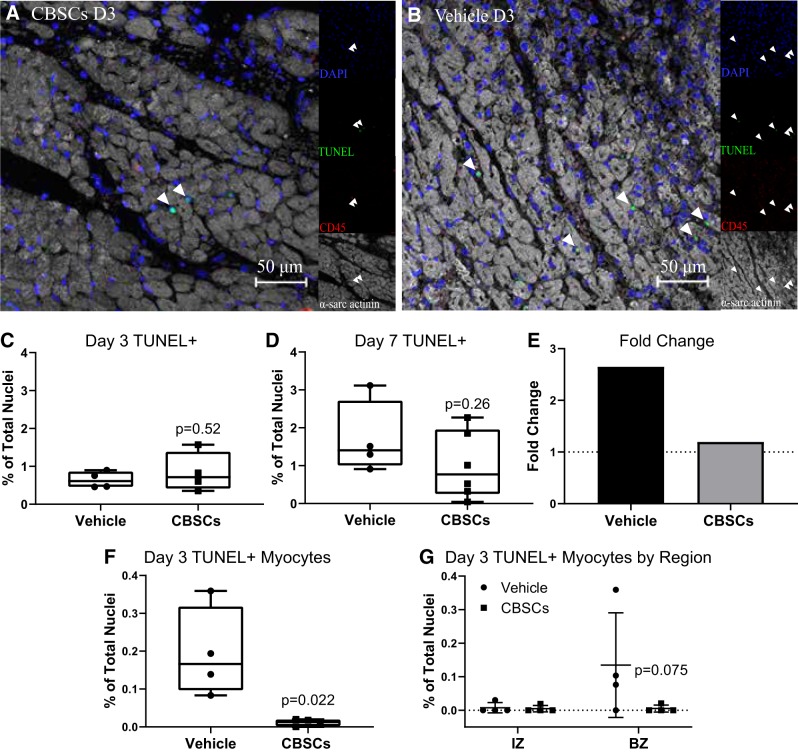
To assess apoptosis at the peak and decline of the inflammatory response, slices from the IZ and adjacent BZ of each heart were stained with TUNEL and costained with fluorescent antibodies to α-sarcomeric actinin and CD45 as well as the nuclear stain DAPI 3 and 7 days after AMI (representative day 3 BZ images Fig. 1, A and B, day 7 Supplemental Fig. 1, https://doi.org/10.6084/m9.figshare.7798688.v1).
Fig. 1.
CBSC treatment reduces apoptosis in the infarcted myocardium. Quantification of fluorescent staining of swine tissue. Representative border zone (BZ) images of the immunofluorescence staining at day 3 (D3) (A and B). Average total TUNEL+ cell counts in the IZ and BZ of CBSC- and vehicle-treated animals 3 days (C) and 7 days (D) after reperfusion were counted by colocalization of TUNEL and DAPI. Fold change (E) reflects the division of the day 7 mean by the day 3 mean for each group. Dotted line represents y = 1. TUNEL+ myocytes were measured by the colocalization of TUNEL and DAPI completely within intact α-sarcomeric actinin+ myocytes (F and G). Percent TUNEL+ Myocytes are expressed as a percentage of total cells counted. Error bars represent ± SD. n = 4 for C, F, and G. n = 4 (vehicle) and 6 (CBSCs) for D. P values reflect the results of unpaired, 2-tailed t tests of CBSCs vs. vehicle (C–F.). For G, P value reflects an ANOVA of CBSCs vs. vehicle in the IZ and BZ with Sidak’s correction for multiple comparisons. CBSC, cortical bone stem cell; IZ, infarct zone; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Overall, the CBSC-treated hearts had fewer cells as represented by the total number of nuclei/mm after 7 days of reperfusion (Supplemental Fig. 8B, vehicle = 2,600 ± 124.1, CBSC = 2,338 ± 88.45, P = 0.04, n = 3/group, https://doi.org/10.6084/m9.figshare.7798688.v1), whereas there was no significant difference in total cells between groups at 3 days of reperfusion (Supplemental Fig. 8A, vehicle = 2,317 ± 243.1, CBSC = 2,211 ± 355.4, P = 0.68, n = 4/group, https://doi.org/10.6084/m9.figshare.7798688.v2). Three days after AMI, there was no difference in the total number of TUNEL+ nuclei as a percent of total nuclei counted in CBSC-treated compared with vehicle-treated animals (Fig. 1C, vehicle = 0.64 ± 0.22%, CBSCs = 0.84 ± 0.052%, P = 0.52, n = 4 per group). At 7 days, however, there was a trend toward fewer total TUNEL+ nuclei in the CBSC-treated group (Fig. 1D, vehicle = 1.7 ± 0.9%, CBSCs = 1.0 ± 0.8%, P = 0.26). Importantly, this change represents a 2.65-fold increase in mean number of apoptotic cells from days 3 to 7 in vehicle-treated pigs versus a 1.19-fold increase in CBSC-treated animals (Fig. 1E). These data indicate CBSCs may be altering the dynamics of apoptosis in the infarcted heart, and the fold-change data may be indicative an inhibition in the increase of apoptosis over time in CBSC-treated hearts.
Apoptotic myocytes were found in all zones of the tissue 3 days after AMI, and there were fewer TUNEL+ myocytes as a percentage of total nuclei in CBSC-treated animals (Fig. 1F, vehicle = 0.19 ± 0.12%, CBSCs = 0.011 ± 0.009%, P = 0.023, n = 4/group). The BZ region was the primary site of myocyte apoptosis, with virtually no myocyte apoptosis detectable in the IZ (Fig. 1G, vehicle BZ = 0.13 ± 0.16%, CBSC BZ = 0.005 ± 0.01%, P = 0.075 by ANOVA, n = 4/group). In addition, no apoptotic myocytes were found in the BZ or IZ of the tissue at 7 days post-AMI.
Myocyte apoptosis accounted for ~2% of the total apoptosis (data not shown) in the infarcted myocardium and less than 0.2% of the total nuclei, and although CBSCs tended to reduce myocyte apoptosis at 3 days, it is unlikely that this small difference in myocyte death accounts for the broad structural and functional changes seen in our previously published studies in this model (28).
CBSCs do not alter total CD45+ cell number but increase macrophage and T-cell presence after 7 days of reperfusion.
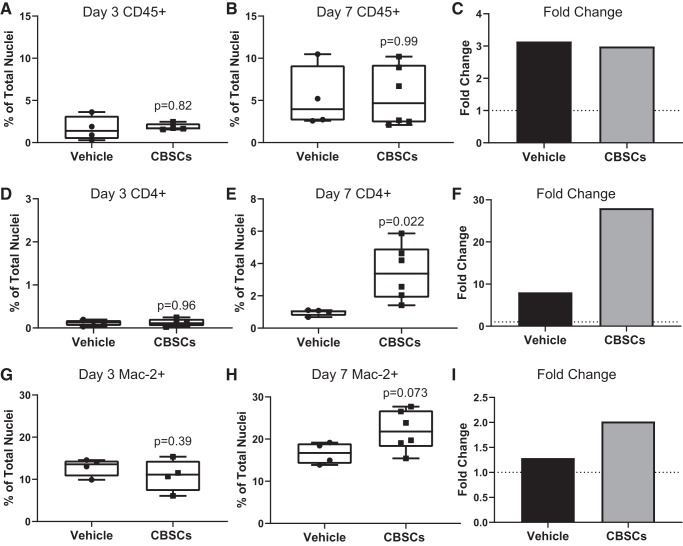
CBSCs possess unique immunomodulatory properties that may interact with immune cell infiltration and recruitment to the infarct region. Inflammatory signals can directly trigger apoptosis in cardiomyocytes and other cardiac cells through caspase-dependent pathways like inflammasome activation, and the clearance of dead cells by phagocytic immune cells is integral to proper wound healing and scar formation. To test whether CBSCs were influencing the overall number of immune cells in the infarcted myocardium, we analyzed the same tissue from Fig. 1 for CD45+ cell presence. CBSCs did not change the dynamics by which CD45+ cells appeared in the tissue over time but did change the makeup of the immune cell population as a whole. There was no change in the number of CD45+ cells in the infarcted myocardium of CBSC-treated animals as a percentage of total nuclei after 3 days of reperfusion [Fig. 2A, vehicle = 1.67 ± 1.45%, CBSCs = 1.84 ± 0.4%, P = 0.83, n = 4 (vehicle) and n = 6 (CBSCs)] or after 7 days of reperfusion [Fig. 2B, vehicle = 5.25 ± 3.7%, CBSCs = 5.50 ± 3.6%, P = 0.91, n = 4 (vehicle) and n = 6 (CBSCs)]. This accounted for a 2.9-fold change from 3 to 7 days in the CBSC group and a 3.14-fold change in the vehicle group (Fig. 2E). The number of apoptotic CD45+ cells was not different between groups as a percentage of total CD45+ cells after 3 days of reperfusion (Supplemental Fig. 8D, vehicle = 15.10 ± 11.5%, CBSC = 12.72 ± 0.66%, P = 0.69, n = 4/group) or after 7 days of reperfusion [Supplemental Fig. 8E, vehicle = 11.8 ± 11.93%, CBSC = 9.39 ± 2.7%, P = 0.64, n = 4 (vehicle) and n = 6 (CBSCs)].
Fig. 2.
CBSCs increase macrophage and T-cell recruitment but not CD45+ cell number. Quantification of the total number of CD45+ cells in the IZ and BZ of CBSC- and vehicle-treated animals (representative images from Fig. 1) 3 days (A) and 7 days (B) after reperfusion. Cells were counted by colocalization of CD45 and DAPI. Percentage of CD4+ cells as a percent of total nuclei counted at 3 days (D) and 7 days (E) after reperfusion were counted by colocalization of CD4 and DAPI. Percentage of Mac-2+ cells as a percent of total nuclei counted at 3 days (G) and 7 days (H) after reperfusion were counted by colocalization of Mac-2 and DAPI. Fold change (C, F and I) reflects the division of day 7 means by day 3 means for each group. Dotted line represents y = 1. Error bars represent ± standard deviation. n = 4 for A, D, and G. n = 4 (vehicle) and 6 (CBSCs) for B, E, and H. P values reflect the results of unpaired, 2-tailed t tests of CBSCs vs. vehicle. BZ, border zone; CBSC, cortical bone stem cell; IZ, infarct zone.
Analysis of CD45 marker expression in the tissue provides information on hematopoietic-derived cells, the vast majority of which are immune cells at these time points. However, this general marker does not indicate the types of immune cells present in the tissue. To assess whether CBSCs were influencing the types of immune cells that were being recruited to the myocardium, we stained concurrent slides with the macrophage marker Mac-2 (galectin-3) and the T-helper cell marker CD4 (Supplemental Figs. 4–7 URL: https://bit.ly/2XipbzH ). CD4+ cells made up ~0.12% of total DAPI+ cells counted for both groups at 3 days of reperfusion (Fig. 2D). After 7 days of reperfusion however, CBSC-treated animals had significantly more CD4+ T cells as a percentage of total DAPI+ cells than vehicle-treated animals and comprised over 3% of the total cells counted [Fig. 2E, vehicle = 0.97 ± 0.19%, CBSCs = 3.45 ± 1.7%, P = 0.022, n = 4 (vehicle) and n = 6 (CBSCs)]. CD4+ cells were 28-fold higher from days 3 to 7 in the CBSC group versus 8-fold higher in the vehicle group (Fig. 2F), representing a large, time-related change in T-cell infiltration dynamics after reperfusion.
After 3 days of reperfusion, CBSC-treated animals had the same number of Mac-2+ macrophages as a percentage of total cells as vehicle-treated animals [Fig. 2G, vehicle = 12.9 ± 2.1%, CBSC = 10.92 ± 3.8%, P = 0.39, n = 4 (vehicle) and n = 6 (CBSCs)]. However, after 7 days, there was a strong trend toward more Mac-2+ macrophages in CBSC-treated animals [Fig. 2H, vehicle = 16.6 ± 2.59%, CBSC = 22.04 ± 4.76%, P = 0.073, n = 4 (vehicle) and n = 6 (CBSCs)]. The percentage of macrophages in the CBSC group doubled from days 3 to 7, whereas the vehicle group increased by a relatively stagnant 1.28-fold (Fig. 2I).
Together, these data indicate that although CBSCs are not changing the total number or apoptotic frequency of CD45+ cells in the infarcted myocardium, they are changing the specific types of cells, namely T cells and macrophages, recruited to the site of injury. It is not yet clear what effects these changes have on the wound healing process in the infarcted heart, but both T cells and macrophages play important roles in the clearance of dead cells and the formation of scar tissue.
CBSCs produce soluble factors capable of altering immune cell recruitment and survival.
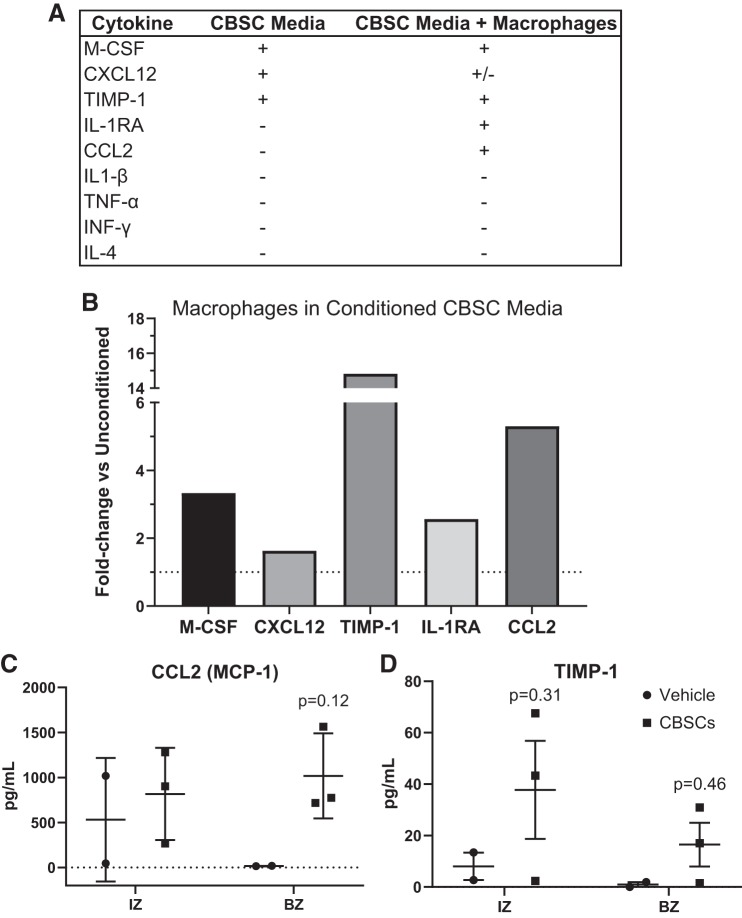
In previous studies, we have shown the CBSCs to be engrafted in the tissue 3 days after injection in conjunction with a lack of effect on pump function or scar size (28). In this study, we confirmed the presence of CBSCs in the tissue at 7 days, again in conjunction with a lack of effect on pump function or scar size (Supplemental Fig. 2, A–D). With the CBSCs still in the tissue at 7 days, we sought to explore whether or not CBSCs were capable of exerting changes over specific immune cell recruitment. To do this, we began by probing the cytokine production of CBSCs in vitro. We have previously shown differences in the swine CBSC gene expression of several immunomodulatory genes compared with cardiac-derived stem cells and mesenchymal stem cells (22). To expand on these data, we profiled the culture media of equivalent mouse CBSCs. Profiling the media using a fluorescent-tagged dot blot and subsequent densitometry, we found the most prominent inflammatory-relevant cytokines were the hematopoietic cell growth factor macrophage-colony stimulating factor (M-CSF), the lymphocyte chemoattractant C-X-C motif chemokine ligand 12 [CXCL12; also known as stromal cell-derived factor 1 (SDF1)], and the antiapoptotic TIMP-1 (Fig. 3A, full profile in Supplemental Fig. 9, https://doi.org/10.6084/m9.figshare.7798688.v1). Given the change in macrophage number in vivo, we sought to assess whether or not CBSC-conditioned media could exert changes on macrophage cytokine production and phenotype in vitro. To test this, we cultured bone marrow-derived macrophages (BMDMs) and exposed them to either unconditioned CBSC media (unused, no cells grown) or conditioned CBSC media (2.5 × 105 cells, 10-mm dish with 8 mL media, 48 h incubation). To ensure any changes to the cytokine profile resulted from CBSC-produced factors and not any factor of the growth medium itself, we analyzed the fold change between macrophages exposed to conditioned and unconditioned media instead of the pixel density alone. When compared with the unconditioned media, there was a 3.3-fold increase in M-CSF, a 14.8-fold increase in TIMP-1, and a 1.63-fold increase in CXCL12. These factors can be said to originate from the CBSCs. In addition, the exposure of BMDMs to conditioned media resulted in a 5.3-fold increase in the production of CCL2 [or monocyte chemoattractant protein 1 (MCP-1)], and a 2.6-fold increase in the anti-inflammatory cytokine IL-1RA (Fig. 3B, full profile in Supplemental Fig. 10. These data indicate that CBSCs produce soluble factors, like M-CSF and CXCL12, shown by others to alter the kinetics of immune cell recruitment and modify the properties of the immune cells recruited to the myocardium. The data also indicate that CBSCs can trigger the production of the macrophage and lymphocyte chemoattractant CCL2 as well as the anti-inflammatory cytokine IL-1RA in macrophages in vitro. In addition, CBSCs without stimulation secrete a potent antiapoptotic signal, TIMP-1.
Fig. 3.
CBSCs secrete soluble signals that can alter immune cell recruitment. Soluble factors in media exposed to CBSCs for 48 h were analyzed by fluorescent antibody-based dot blot and densitometry, shown in a representative list (A). Densitometry values were obtained for macrophages exposed to conditioned or unconditioned CBSC media for 24 h, and the average values for each marker in the conditioned media group was divided by the average values for the unconditioned media group to obtain the fold change between groups (B). Dotted line represents y = 1. Quantitative proteomics on snap-frozen heart tissue 7 days after AMI was performed (C and D). Error bars represent ± SD. n = 3 for CBSC and n = 2 for vehicle groups. P values reflect the results of a 2-way ANOVA interactions between CBSC and vehicle groups. Multiple comparisons were corrected for by using Sidak’s method for multiple comparisons. AMI, acute myocardial infarction; BZ, border zone; CBSC, cortical bone stem cell; CCL2, chemokine C-C motif ligand 2; CXCL-12, C-X-C motif chemokine ligand 12; IL-1RA, interleukin-1 receptor antagonist; IZ, infarct zone; MCP-1, monocyte chemoattractant protein 1; M-CSF, macrophage-colony stimulating factor; TIMP-1, tissue inhibitor of metalloproteinase metallopeptidase inhibitor 1.
To confirm these finding in vivo, we used frozen tissue punch biopsies from the IZ and BZ of the pigs receiving 7 days of reperfusion after AMI and performed quantitative proteomics for TIMP-1, CCL2, IL-1RA, and IL-1β. CCL2 [Fig. 3C, BZ vehicle = 16.5 pg/mL ± 2.7, BZ CBSC = 1,018.9 pg/mL ± 473.1, P = 0.12, n = 3 (CBSCs) and n = 2 (vehicle)] trended toward increased in the BZ of the infarct region in CBSC-treated animals, and there was a trend toward increased TIMP-1 in the BZ of CBSC-treated animals (Fig. 3D, BZ vehicle = 0.95 ± 1.74, BZ CBSC = 16.0 ± 21.1, P = 0.46, n = 3/group). Neither IL-1β nor IL-1RA was detected above background levels at 7 days post-AMI. These findings indicate that cytokine changes brought about as a result of CBSC treatment in vitro can occur in vivo and imply that CBSCs may be altering the signals involved in cell recruitment and survival post-AMI. Tests for M-CSF and CXCL12 were not available for swine and so were not tested.
DISCUSSION
The goal of this study was to assess the effect of CBSC treatment on myocytes and nonmyocytes in a translational large animal model of AMI and correlate changes in apoptosis with changes in immune cell profile. In previous studies, we showed pump function improvement and decreased scar size in this large animal model (28). However, it was apparent from those studies that neither the production of new myocytes nor the prevention of myocyte death was robust enough to adequately explain the changes in gross anatomical scar size and LV pump function after CBSC treatment. In addition, the CBSCs were present in the tissue 3 days after injection but not 3 mo later, indicating an effect that outlasted the presence of the CBSCs in the tissue. Through this study, we have gained insight into the effects of CBSCs on not only the cardiomyocyte population but also noncardiomyocyte and immune cell populations including macrophages and T cells that play crucial roles in infarct healing. There were several important findings: intramyocardial injections of CBSCs within 2 h after infarction resulted in CBSC engraftment and suggested a decrease in the frequency of apoptosis overall 7 days after AMI (Fig. 1, C–E, Supplemental Fig. 2). In addition, there was a decrease in myocyte apoptosis overall and a trend toward decreased myocyte apoptosis in the BZ specifically of CBSC-treated myocardium (Fig. 1, F and G). CD45+ cell infiltration in the myocardium was the same in CBSC-treated hearts compared with vehicle-treated hearts (Fig. 2, A–C). T cell and macrophage recruitment to the tissue was increased in CBSC-treated animals (Fig. 2, D–I), and CBSCs produce cytokines and growth factors that are known to promote T cell and macrophage growth, chemotaxis, differentiation, and survival (Fig. 3, A–D). In addition, these factors appear to promote the secretion of anti-inflammatory and chemoattractant cytokines by BMDMs in vitro and in vivo (Fig. 3, A–D).
CBSCs inhibit apoptosis in the infarcted myocardium.
Some researchers have questioned the relevance of cardiomyocyte apoptosis in AMI and have put forth evidence that cardiomyocytes do not possess the cellular machinery to undergo apoptosis at all (14, 27). In the present study, we detected apoptotic myocytes at 3 days in every zone and a very small number of apoptotic myocytes at 7 days exclusively in the remote zone. It is most likely that necrosis of myocytes in the infarct core is the major contributor to LV dysfunction after AMI. However, it is still unclear whether or not reducing the already small number of apoptotic myocytes over a longer time period has effects on overall LV function after AMI. Our previous studies in large animals have shown no difference in overall LV function at 3 days, supporting the notion that a difference in myocyte apoptosis may not translate to functional differences at this short time point (28). It is important to note that apoptosis is a relatively rapidly occurring cellular process, and our analysis captures a snapshot of the immense cellular changes happening over this time period. With this in mind, myocytes undergoing apoptosis in the days following AMI may account for larger changes in function than we can measure with our current techniques, and preventing them from dying over the course of days and weeks could have long-term functional effects. Our data from the present study show very few apoptotic myocytes at 7 days of reperfusion, and only in the remote zones of the tissue away from the infarction, underlining the “backseat” role that myocyte apoptosis may take in the days immediately following AMI. However, these data also serve to highlight the importance of the contributions of nonmyocytes, such as immune cells, on infarct healing and pump function.
Interestingly, our data show that although CBSCs may confer some protection from myocyte apoptosis 3 days after AMI, there is only a trend toward a decrease in apoptosis overall after 7 days of reperfusion. Since no apoptotic myocytes were detected at 7 days in the infarct and BZ, CBSCs must be having an effect on other cell types. CD45+TUNEL+ cells accounted for ~33% of total TUNEL+ cells at 7 days, implying that approximately two-thirds of apoptotic cells are of nonhematopoietic origin or take place in immune cells that no longer express CD45. The identity of these cells remains unknown in this model and will be the subject of future studies.
CBSCs increase macrophage and T-cell recruitment to the infarct.
As previously mentioned, CBSCs are present in the tissue 3 days after injection but not 3 mo (28). The present experiments document that CBSCs are still engrafted within the damaged tissue 7 days after AMI, but no discernible effects on function or scar size were observed at this early time point. If the effects of CBSCs are to outlast their lifespan in the tissue, they must make acute changes to populations of cells that persist weeks and months after AMI. The effects of immune cells targeted to the site of ischemia can be seen long after the resolution of inflammation, and cells like lymphatic CD4+ regulatory T cells (Tregs) and memory T cells can reside in damaged tissue for weeks after injury and exert specific control over tissue repair and maintenance (3). This study did not examine the subtypes of T cells present in the tissue, and confirming the types of T cells present is the aim of future studies.
Modulation of the inflammatory response, within reason, may protect myocytes and other cells that are teetering between survival and death. Although this study did not fully examine the potential for anti-inflammatory modulation by CBSCs in vivo, it began to explore the effects of CBSC signals on macrophages in vitro, demonstrating the potential of CBSCs to induce the release of the anti-inflammatory signal IL-1RA and the chemoattractant CCL2. Our data pertaining to the increase of CD4+ cells and macrophages at 7 days and the slowed progression of apoptosis from 3 to 7 days of reperfusion in CBSC-treated animals, as indicated by the fold change of total apoptosis in Fig. 1E, coincides with the resolution of the inflammatory response and the switch to a prohealing cellular milieu in the infarcted myocardium. Many studies in the mouse have shown that alternatively activated (M2) macrophages and CD4+ Tregs infiltrate the tissue over this time period and play critical roles in the formation of scar and the healing of the infarct (12, 24). This study does not conclusively determine the nature of the CD4+ T cells or macrophages, but if CBSCs are promoting the recruitment of these cells, it may begin to explain the reduced myocardial damage, reduced scar size, and increased LV function seen in these large animals 3 mo after AMI.
CBSCs secrete factors known to influence immune cell survival and differentiation.
To begin to understand how CBSCs may be interacting with the cells around them to slow apoptosis and alter immune cell recruitment and infiltration, we investigated the factors secreted by CBSCs in vitro under baseline conditions. The three factors present in the highest concentrations were M-CSF, CXCL12, and TIMP-1. M-CSF, a classic immune cell growth factor, is known to induce the differentiation of bone marrow cells to macrophages, induce an immunosuppressive phenotype in BMDMs, and induce the production of CCL2 (30). Indeed, CCL2 and IL-1RA were increased in conditioned media-treated BMDMs (Fig. 3B).
TIMP-1 has been known for over a decade to inhibit apoptosis in a number of different cell types, including breast epithelial cells (21), pancreatic islet cells (16), and normal human granulocytes (9). This factor was the most produced of any observed by densitometry and could have potent antiapoptotic effects within this model of AMI. This study did not determine the causative effect of TIMP-1 on heart cells in an AMI model, but the trend of increased TIMP-1 in the BZ of CBSC-treated animals 7 days after AMI correlates with the trend of decreased apoptosis overall at this time point. Further studies are required to examine the exact effects of TIMP-1 in this model.
CXCL12 is a chemoattractant that is produced immediately following AMI and is increased for at least 7 days after AMI (4). Upon upregulation at the site of injury, CXCL12 strongly attracts lymphocytes (6) and has also been shown to protect cardiomyocytes from apoptosis (34). CXCL12 is produced by CBSCs in vitro, and our data show significantly more CD4+ T cells in the myocardium of CBSC-treated animals 7 days after AMI (Fig. 3B). Taken together, these data suggest that CBSCs are capable of recruiting T cells to the site of injury and protecting cardiomyocytes. The role of T cells in myocardial healing has been studied and reviewed (19). However, the potential for stem cell treatment to modulate the T-cell response to injury in ways that improve healing has yet to be studied. Further studies are required to determine if a cause-effect relationships exits between the secreted factors of CBSCs and these target cells.
To begin to test the effects of CBSC factors on innate immune cells, we chose to expose BMDMs to the culture medium in which the CBSCs were grown. We then compared this media (conditioned media) to media from macrophages that had been exposed to stem cell media in which no CBSCs were grown (unconditioned media). The fold change of cytokine production was determined by comparing densitometry values. In accordance with the screen of CBSC media alone, M-CSF, CXCL12, and TIMP-1 were all increased compared with unconditioned media. In addition to these factors, two others were increased that were not present in CBSC media alone: CCL2 (also known as MCP-1) and IL-1RA. CCL2 is a potent chemotactic signal for monocytes and lymphocytes, including memory and regulatory T cells (8), and plays a crucial role in the healing of the infarct (10). CCL2 is potentially increased compared with vehicle-treated hearts in the BZ tissue 7 days after AMI and may begin to explain the increased CD4+ and Mac-2+ cell populations at this time. In addition, IL-1RA is the direct antagonist to the inflammatory cytokine IL-1β, which has been shown to induce apoptosis in myocytes specifically (29). However, IL-1β production and signaling occurs shortly after the beginning of ischemia and is thought to be relatively transient, peaking within 2 h of the onset of ischemia in humans (15). This is consistent with our findings of no IL-1β or IL-1RA in the tissue at 7 days after AMI, a time point that may be beyond the limits of the production of these cytokines. Further investigation is needed to determine the individual effects of CBSC cytokine production on immune cell phenotypes as a whole.
Study limitations.
This study was performed with one stem cell type, using one delivery method and a single animal model. Using a large animal model of AMI provides many benefits over smaller animal and rodent models and is more translational in nature. However, complexity and cost of the model dictated the number of animals that could be used per group for a given study. Given this, we are cautious in our inferences from these data because of the small sample size of this study. In addition, the animals used in this study were relatively young and without any comorbidities, neither of which are common in the clinical patient pool of AMI. The results presented in this paper incite probing questions into the nature of stem cell therapy and the potential for the modulation of the innate and adaptive immune response to injury as a path toward improved cardiac wound healing.
There are technical limitations to the histological analysis of large organs like the pig heart as well. Analysis of slices of tissue gives a snapshot of that region of tissue, and it is not feasible to analyze the entirety of the organ. TUNEL staining also depends on the exposure of broken DNA strands by Proteinase K digestion and is sensitive to over- and under-digestion. CD45, CD4, and Mac-2 staining was done by antibody labeling and could possibly have some off-target effects. These factors were controlled for in every way possible according to the manufacturer’s protocols.
Confocal microscopy also comes with its own technical limitations. The colocalization of DAPI, TUNEL, CD45, and α-sarcomeric actinin was determined by analysis software and checked by the researcher analyzing the images. However as with all histological analysis, the three-dimensional nature of tissue sections can lead to accidental identification of cell types by inadvertent overlapping of cells.
Conclusions.
Our examination of CBSC therapy and apoptosis in a swine model of AMI shows the following: 1) CBSC treatment may decrease global apoptosis in and around the infarct after 7 days of reperfusion; 2) CBSCs significantly decrease myocyte apoptosis; 3) The decrease in apoptosis in CBSC-treated animals compared with vehicle-treated animals after 7 days of reperfusion does not affect CD45+ cells in the tissue; 4) CBSC treatment alters immune cell recruitment and increases the number of macrophages and T cells in the tissue after 7 days; and 5) CBSCs produce soluble factors that have been shown to prevent apoptosis and increase immune cell recruitment. These results raise the question of if and how stem cell therapy is interacting with the immune system and what implications these interactions have on wound healing in the damaged heart. Together, these conclusions support the hypothesis that CBSCs reduce apoptosis in the heart after AMI, and this could lead to the observed reduction in scar size and associated improvement in cardiac pump function.
GRANTS
S. Houser received funding from NIH (R01HL139960), and S. Mohsin received funding from NIH (R56-HL137850, RO1-HL137850) and the American Heart Association (SDG-15SDG25550038). A. Hobby received funding from the American Heart Association (18PRE33960122).
DISCLOSURES
S. R. Houser is a named inventor on intellectual property filings that are related to the cortical bone-derived stem cells used in this study. In addition, S. R. Houser is a cofounder and scientific advisor and holds equity in MyocardTherapeutics, LLC, a biotech startup which will license S. R. Houser’s cortical bone cell technology from Temple University for commercial development and clinical trials. MyocardTherapeutics, LLC, has not funded any aspect of this research.
AUTHOR CONTRIBUTIONS
A.R.H. and T.E.S. conceived and designed research; A.R.H., T.E.S., R.M.B., G.B., and E.F. performed experiments; A.R.H. analyzed data; A.R.H. and S.M. interpreted results of experiments; A.R.H. prepared figures; A.R.H. drafted manuscript; A.R.H. and S.M. edited and revised manuscript; S.M. and S.R.H. approved final version of manuscript.
REFERENCES
- 1.Abbate A, Bussani R, Biondi-Zoccai GGL, Santini D, Petrolini A, Giorgio FD, Vasaturo F, Scarpa S, Severino A, Liuzzo G, Leone AM, Baldi F, Sinagra G, Silvestri F, Vetrovec GW, Crea F, Biasucci LM, Baldi A. Infarct-related artery occlusion, tissue markers of ischaemia, and increased apoptosis in the peri-infarct viable myocardium. Eur Heart J 26: 2039–2045, 2005. doi: 10.1093/eurheartj/ehi419. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med 376: 2053–2064, 2017. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- 3.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A distinct function of regulatory T cells in tissue protection. Cell 162: 1078–1089, 2015. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362: 697–703, 2003. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 5.Bernink FJP, Timmers L, Beek AM, Diamant M, Roos ST, Van Rossum AC, Appelman Y. Progression in attenuating myocardial reperfusion injury: An overview. Int J Cardiol 170: 261–269, 2014. doi: 10.1016/j.ijcard.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med 184: 1101–1109, 1996. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahill TJ, Kharbanda RK. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: mechanisms, incidence and identification of patients at risk. World J Cardiol 9: 396–469, 2017. doi: 10.4330/wjc.v9.i5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA 91: 3652–3656, 1994. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chromek M, Tullus K, Lundahl J, Brauner A. Tissue inhibitor of metalloproteinase 1 activates normal human granulocytes, protects them from apoptosis, and blocks their transmigration during inflammation. Infect Immun 72: 82–88, 2004. doi: 10.1128/IAI.72.1.82-88.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. 96: 881–889, 2005. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 11.Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo H, Houser SR. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res 113: 539–552, 2013. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res 102: 240–248, 2014. doi: 10.1093/cvr/cvu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiu K, Wang J, Nagai R. Cardioprotective function of cardiac macrophages. Cardiovasc Res 102: 232–239, 2014. doi: 10.1093/cvr/cvu059. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Dorado D, Rodriguez-Sinovas A, Ruiz-Meana M, Inserte J. Protection against myocardial ischemia-reperfusion injury in clinical practice. Rev Esp Cardiol (Engl Ed) 67: 394–404, 2014. doi: 10.1016/j.recesp.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Guillen I, Blanes M, Gomez-Lechon MJ, Castell JV. Cytokine signaling during myocardial infarction: sequential appearance of IL-1 beta and IL-6. Am J Physiol Regul Integr Comp Physiol 269: R229–R235, 1995. doi: 10.1152/ajpregu.1995.269.2.R229. [DOI] [PubMed] [Google Scholar]
- 16.Han X, Sun Y, Scott S, Bleich D. Tissue inhibitor of metalloproteinase-1 prevents cytokine-mediated dysfunction and cytotoxicity in pancreatic islets and β-cells. Diabetes 50: 1047–1055, 2001. doi: 10.2337/diabetes.50.5.1047. [DOI] [PubMed] [Google Scholar]
- 17.Hao M, Wang R, Wang W. Cell therapies in cardiomyopathy: current status of clinical trials. Anal Cell Pathol (Amst) 2017: 9404057, 2017. doi: 10.1155/2017/9404057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123: 92–100, 2013. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann U, Frantz S. Role of T-cells in myocardial infarction. Eur Heart J 37: 873–879, 2016. doi: 10.1093/eurheartj/ehv639. [DOI] [PubMed] [Google Scholar]
- 20.Leblond AL, Klinkert K, Martin K, Turner EC, Kumar AH, Browne T, Caplice NM. Systemic and cardiac depletion of M2 macrophage through Csf-1r signaling inhibition alters cardiac function post myocardial infarction. PLoS One 10: e0137515, 2015. doi: 10.1371/journal.pone.0137515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res 59: 6267, 1999. [PubMed] [Google Scholar]
- 22.Mohsin S, Troupes CD, Starosta T, Sharp TE, Agra EJ, Smith S, Duran JM, Zalavadia N, Zhou Y, Kubo H, Berretta RM, Houser SR. Unique features of cortical bone stem cells associated with repair of the injured heart. Circ Res 117: 1024–1033, 2015. doi: 10.1161/CIRCRESAHA.115.307362. [DOI] [PubMed] [Google Scholar]
- 23.Murray CJ, Barber RM, Foreman KJ, Ozgoren AA, Abd-Allah F, Abera SF, Aboyans V, Abraham JP, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Achoki T, Ackerman IN, Ademi Z, Adou AK, Adsuar JC, Afshin A, Agardh EE, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allebeck P, Almazroa MA, Alsharif U, Alvarez E, Alvis-Guzman N, Amare AT, Ameh EA, Amini H, Ammar W, Anderson HR, Anderson BO, , et al. . Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 386: 2145–2191, 2015. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ndrepepa G, Colleran R, Kastrati A. Reperfusion injury in ST-segment elevation myocardial infarction: the final frontier. Coron Artery Dis 28: 253–262, 2017. doi: 10.1097/MCA.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 26.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 56: 786–794, 1977. doi: 10.1161/01.CIR.56.5.786. [DOI] [PubMed] [Google Scholar]
- 27.Sanchis D, Llovera M, Ballester M, Comella JX. An alternative view of apoptosis in heart development and disease. Cardiovasc Res 77: 448–451, 2008. doi: 10.1093/cvr/cvm074. [DOI] [PubMed] [Google Scholar]
- 28.Sharp TE 3rd, Schena GJ, Hobby AR, Starosta T, Berretta RM, Wallner M, Borghetti G, Gross P, Yu D, Johnson J, Feldsott E, Trappanese DM, Toib A, Rabinowitz JE, George JC, Kubo H, Mohsin S, Houser SR. Cortical bone stem cell therapy preserves cardiac structure and function after myocardial infarction. Circ Res 121: 1263–1278, 2017. doi: 10.1161/CIRCRESAHA.117.311174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Y, Qin J, Bu P. Pathways involved in interleukin-1β-mediated murine cardiomyocyte apoptosis. Tex Heart Inst J 42: 109–116, 2015. doi: 10.14503/THIJ-14-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol 100: 481–489, 2016. doi: 10.1189/jlb.3RU0316-144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J 28: 2667–2677, 2007. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 32.Veinot JP, Gattinger DA, Fliss H. Early apoptosis in human myocardial infarction. Hum Pathol 28: 485–492, 1997. doi: 10.1016/S0046-8177(97)90039-3. [DOI] [PubMed] [Google Scholar]
- 33.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 21: 3197–3207, 2007. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]