Abstract
Abnormally high epithelial Na+ channel (ENaC) activity in the aldosterone-sensitive distal nephron and collecting duct leads to hypertension. Myelin and lymphocyte (Mal) is a lipid raft-associated protein that has been previously shown to regulate Na+-K-2Cl− cotransporter and aquaporin-2 in the kidney, but it is not known whether it regulates renal ENaC. ENaC activity is positively regulated by the anionic phospholipid phosphate phosphatidylinositol 4,5-bisphosphate (PIP2). Members of the myristoylated alanine-rich C-kinase substrate (MARCKS) family increase PIP2 concentrations at the plasma membrane, whereas hydrolysis of PIP2 by phospholipase C (PLC) reduces PIP2 abundance. Our hypothesis was that Mal protein negatively regulates renal ENaC activity by stabilizing PLC protein expression at the luminal plasma membrane. We investigated the association between Mal, MARCKS-like protein, and ENaC. We showed Mal colocalizes with PLC-β3 in lipid rafts and positively regulates its protein expression, thereby reducing PIP2 availability at the plasma membrane. Kidneys of 129Sv mice injected with MAL shRNA lentivirus resulted in increased ENaC open probability in split-open renal tubules. Overexpression of Mal protein in mouse cortical collecting duct (mpkCCD) cells resulted in an increase in PLC-β3 protein expression at the plasma membrane. siRNA-mediated knockdown of MAL in mpkCCD cells resulted in a decrease in PLC-β3 protein expression and an increase in PIP2 abundance. Moreover, kidneys from salt-loaded mice showed less Mal membrane protein expression compared with non-salt-loaded mice. Taken together, Mal protein may play an essential role in the negative feedback of ENaC gating in principal cells of the collecting duct.
Keywords: epithelial Na+ channel; kidney; myelin and lymphocyte; phosphatidylinositol 4,5-bisphosphate; phospholipase C
INTRODUCTION
Myelin and lymphocyte (Mal) proteins comprise two highly conserved family members: Mal and Mal2 (11, 14, 27). Both Mal and Mal2 are tetraspanning membrane proteins that associate with lipid rafts in most cell types (39). Mal is associated with glycosphingolipids and believed to participate in the transport, organization, and maintenance of apical proteins within detergent-resistant microdomains (10). Zacchetti et al. (56) first showed that Mal is involved in vesicular trafficking between the Golgi apparatus and apical plasma membrane. Although Mal is not ubiquitously expressed, it is abundant at the luminal plasma membrane of renal epithelial cells, including the thick ascending limb, distal tubule, and collecting duct (15). However, Mal protein can do more than act as a trafficking chaperone; Mal protein has also been previously shown to directly associate with and stabilize renal-specific Na+-K+-2Cl− cotransporter (NKCC2) at the apical plasma membrane (8), promote collecting duct ciliogenesis and cilia formation (45), and increase aquaporin-2 (AQP2) density at the surface membrane (22). Therefore, we speculated that Mal might be involved in stabilizing other membrane proteins involved in apical membrane Na+ transport. The epithelial Na+ channel (ENaC) is an apical membrane Na+ transporter that is responsible for fine tuning Na+ handling in the distal tubule and collecting duct (36). Activity of ENaC requires proteases to cleave the α- and γ-subunits of ENaC (αENaC and γENaC) in the trans-Golgi or after insertion in the apical membrane (5, 17, 18, 20, 32, 33, 46, 47). Once in the membrane and activated by cleavage, ENaC activity requires an interaction with an anionic phosphatidyl inositol lipid phosphate, usually phosphatidylinositol 4,5-bisphosphate (PIP2) (23–26, 37, 55, 57). Delivery of PIP2 to the cytosolic NH2- and COOH-termini of ENaC is determined by the chaperone protein myristoylated alanine-rich C-kinase substrate (MARCKS) or its kidney-specific isoform, MARCKS-like protein 1 (MLP1). βENaC (3) and possibly γENaC (37) are mainly responsible for binding anionic phospholipid phosphates, including PIP2, and maintaining the channel in an open confirmation.
Phospholipase C (PLC) plays an essential role in the generation of diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) from the hydrolysis of PIP2. Cha et al. (9) previously identified the distribution of PLC enzymes in the rat kidney. Moreover, Mazuruk et al. (29) showed that PLC-β3 mRNA expression is highly expressed in the kidney. However, the putative regulation of PLC by Mal protein and its effect on ENaC activity have not been studied.
The overall goal of the present study was to investigate the regulation of renal ENaC by Mal protein. One aim was to determine whether Mal protein regulates ENaC gating by modulating PIP2 availability at the apical plasma membrane. A second aim was to examine Mal protein regulation in the salt-sensitive kidney where ENaC contributes to blood pressure regulation. We performed overexpression and knockdown experiments to investigate the molecular mechanism by which Mal stabilizes PLC-β3 protein expression to alter PIP2 availability and thus ENaC membrane expression and activity. To our knowledge, this is the first report of Mal protein regulating ENaC in polarized epithelial cells of the kidney.
MATERIALS AND METHODS
Cell culture.
Mouse cortical collecting duct (mpkCCDc14) principal cells were plated on 24-mm Transwell permeable supports (Corning) and maintained in 5% CO2 at 37°C. Culture media consisted of 50 nM dexamethasone, 1 nM triiodothyronine, 20 mM HEPES, 2 mM l-glutamine, 0.1% penicillin-streptomycin, and 2% heat-inactivated FBS. Only low-passage cells were used for experiments.
Animals.
All animal studies were approved by University of Florida’s Institutional Animal Care and Use Committee and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult 9- to 12-mo-old male and female 129Sv (129X1/SvJ) and C57B6 wild-type mice originally purchased from Jax Laboratories were salt loaded and maintained on a 4% NaCl diet for 14 days. Isoflurane was administered before the injections of the shRNA virus. All animals were euthanized between 4 and 6 PM at the end of the study.
Mammalian two-hybrid and dual-luciferase reporter assay.
To construct the pBIND-MLP and pACT-MAL plasmids, rat MLP and MAL cDNA were cloned by RT-PCR from pooled rat kidney cDNA. Primers were designed based on available sequences while incorporating Sal1 and Xba1 restriction sites to allow for cloning of full-length fragments of MLP and MAL into the pBIND and pACT vectors. For each condition, three inserts were cotransfected with 8 μg of each plasmid as described using the Xfect transfection reagent. mpkCCD cells were cotransfected with the pG5-luciferase vector in the presence or absence of pBIND-MAL with or without pACT-MLP1. A second set of cells was cotransfected with the pG5-luciferase vector along with either pBIND and pACT as a negative control or pBIND-ID and pACT-MyoD as a positive control. Another set of cells was cotransfected with the pG5-luciferase vector and pBIND-MAL and pACT-ENaCα or pBIND-ENaCγ and pACT-MLP1. Cells were cultured for 72 h at 37°C after transfection before being harvested for protein. Renilla luciferase activity from the pBIND plasmid was used to correct for transfection efficiencies.
Transient transfections.
At 70% confluence, mpkCCD cells cultured on six-well permeable supports were initially transfected with 5 μg plasmid DNA [Mal-yellow fluorescent protein (YFP), MARCKS wild type, or MARCKS S3A (third serine residue in the effector domain mutated to an alanine)] per well using the Xfect transfection reagent (Clontech Laboratories) according to the manufacturer’s instructions but with the following modifications: a volume of 500 μl cell culture media was applied to the apical side of the cells while cells were incubated with the transfection reagent and plasmid DNA. Cells were transfected again 24 h after the initial transfection using the same amount of plasmid DNA before being cultured for an additional 72 h. Fluorescence and Western blot analysis were used to determine transfection efficiency.
Immunoprecipitation.
In some experiments, mpkCCD cells were transiently transfected with wild-type MLP1 or mutant MLP1. Two hundred micrograms of total protein lysed in RIPA buffer supplemented with protease and phosphatase inhibitors (Thermo Scientific) were incubated with 1:250 dilution of anti-Mal polyclonal antibody at 4°C for 4 h with end-over-end mixing. The resulting complex was incubated with 1:10 dilution of prewashed 50% protein G agarose (Millipore) at 4°C for 6 h with end-over-end mixing. The beads were washed three times with ice-cold RIPA buffer, and the bound proteins were eluted in 1× SDS sample buffer before being analyzed by SDS-PAGE and Western blott analysis.
SDS-PAGE and Western blot analysis.
The BCA protein assay (ThermoFisher Scientific) was used to determine protein concentration. Fifty micrograms of total protein from cellular or tissue lysates prepared in Laemmli sample buffer were loaded onto 4–20% Tris·HCl polyacrylamide gels, and proteins were resolved on the Criterion electrophoresis system (Bio-Rad). Proteins were electrically transferred to nitrocellulose blotting membranes (ThermoFisher Scientific) using the Criterion transfer system (Bio-Rad). Membranes were blocked in 5% nonfat milk in 1× Tris-buffered saline (TBS; Bio-Rad) for 1 h at room temperature, washed twice with 1× TBS for 4-min intervals, and then incubated with the following validated primary antibodies: ENaCα (3), ENaCβ (3), ENaCγ (28), Mal (catalog no. PA1-085, Thermo Scientific), PLC-β3 (catalog no. 2482, Cell Signaling), flotillin-2 (catalog no. 3244, Cell Signaling), and mu-2 (1) antibody at dilution of 1:1,000 in 5% (wt/vol) BSA in 1× TBS while on a rocker at 4°C overnight. Membranes were washed three times with 1× TBS for 4-min intervals and then incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody at dilution of 1:3,000 prepared in blocking solution for 1 h. Membranes were washed four times with 1× TBS for 4-min intervals, incubated with SuperSignal Dura Chemiluminescent Substrate for 7 min, and then imaged on a Bio-Rad ChemiDoc MP Imaging System with Image Lab software.
Sucrose density gradient assays.
Lipid rafts were isolated from mpkCCD cell lysates by sucrose density gradient ultracentrifugation, as previously described (30), and Mal protein expression in these fractions and in nonlipid raft fractions was assessed by Western blot analysis using Mal-specific antibody.
In vivo shRNA knockdown of renal Mal protein.
Lentivirus encoding mCherry or mCherry-MAL shRNA sequence were produced by Emory’s Viral Vector Core. In vivo virus transduction to express MAL shRNA in mouse kidneys was performed as previously described by Nakamura et al. (31) with slight modifications. In anesthetized mice, after temporary occlusion of the left renal pedicle, a 31-gauge needle was inserted at the lower pole of the left kidney parallel to the long axis and was carefully pushed toward the upper pole. As the needle was slowly removed, 100 µl filter-purified lentivirus cocktail (∼5 × 104 IU/µl) or saline was injected. Lentivirus-mediated mCherry or mCherry-MAL shRNA expression in the kidney parenchyma (detected by Western blot analysis) was robust after 48 h, and other investigators have shown that expression persists from 5 wk to 2 mo (12).
Single channel patch clamp.
Renal tubules were manually dissected, and the cortical collecting duct was identified by morphology. Tubules were placed in a physiological saline solution [containing (in mM) 140 NaCl, 5 KCl, 1 CaCl2, and 10 HEPES adjusted to pH 7.4 with NaOH] in a plastic dish before being split open to reveal the apical surface of the cells before single channel patch clamp, as previously described for patch clamp of cells in culture (7, 43, 52–54). Briefly, a microelectrode was filled with physiological buffer solution in which Li+ was substituted for Na+ [containing (in mM) 140 LiCl, 5 KCl, 1 CaCl2, and 10 HEPES adjusted to pH 7.4 with NaOH] and lowered to a single cell before application of a small amount of suction to achieve a >10 GΩ seal. ENaC channels were identified by characteristic channel kinetics (long mean open and closed times > 0.5 s) and the current-voltage relationship of the channel (unit conductance close to 6 pS and a very positive, >40-mV, reversal potential).
Immunofluorescence.
For overexpression experiments, mpkCCD cells were transfected with MAL-YFP using Xfect reagent 72 h before being incubated and labeled with 1 μg/ml cholera toxin B-subunit conjugated to Alexa 594 (Molecular Probes) in 0.1% BSA-PBS for 30 min at 4°C. Fluorescence was examined using an Olympus FluoView FV1000 confocal microscope. For knockdown experiments, mpkCCD cells were transfected with MAL siRNA or control siRNA when cells were 70–90% confluent and then again 24 h later. Cells were harvested for protein 72 h later, and the lysate was used for Western blot analysis. In other experiments, cells transfected twice with MAL siRNA or control siRNA were then transfected with the PIP2 reporter green fluorescent protein (GFP)-tagged pleckstrin homology domain of PLC-δ. Fluorescence was examined using a Nikon A1RMP confocal microscope.
Amiloride-sensitive transepithelial current measurements.
An epithelial volt-ohm-meter 2 (World Precision Instruments, Sarasota, FL) equipped with chopstick electrodes was used to measure transepithelial voltages and transepithelial resistances across confluent cells that formed tight junctions. Amiloride-sensitive transepithelial current was determined by applying 0.5 μM amiloride to the apical side of cells before transepithelial current (Ite) was calculated from Ohm’s law (Ite = Vte/Rte, where Vte is transepithelial voltage and Rte is transepithelial resistance).
Blood pressure measurements.
Blood pressure in 9- to 12-mo-old male and female 129Sv and C57B6 wild-type mice was measured between 1 and 3 PM at three separate time points for each group of mice maintained on a normal or high-salt diet and after three amiloride injections (1 mg·kg−1·day−1) on separate days between 5 and 6 PM using a mouse tail-cuff IITC MRBP system (Life Science, Woodland Hills, CA).
Kidney homogenate preparation.
Kidney cortex samples from male and female 129Sv and C57B6 mice were snap frozen in liquid nitrogen and stored at −80°C. Fifty milligrams of tissue were homogenized in ice-cold tissue protein extraction reagent (Thermo Scientific) supplemented with protease and phosphatase inhibitors. Samples were centrifuged at 5,000 g to remove debris, and the supernatants were subject to ultracentrifugation for 30 min at 110,000 g. The pellet was reconstituted in tissue protein extraction reagent.
Statistical analysis.
All data are reported as mean values ± SE and were compared using either a Student's t-test or one-way ANOVA with SigmaPlot software (Jandel Scientific). Differences with P values of <0.05 were considered statistically significant.
RESULTS
Association between Mal protein and ENaC or MLP1.
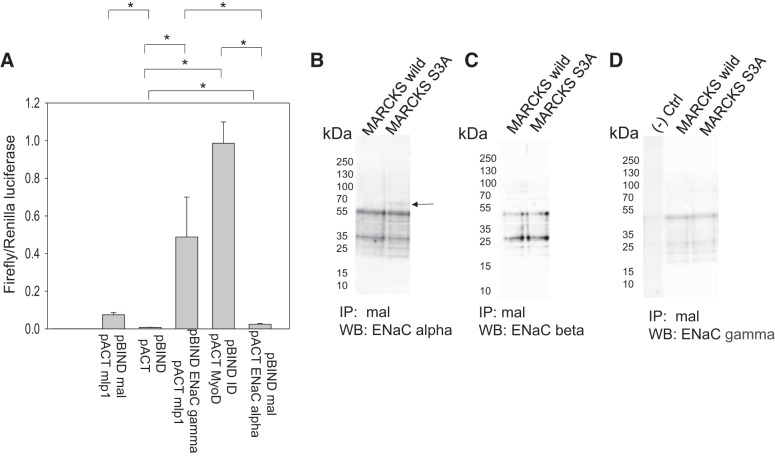
Others have shown that Mal protein directly interacts with transport proteins, including NKCC2 and AQP2 in the kidney (8, 22). Mal protein stabilizes NKCC2 and AQP2 proteins at the apical plasma membrane by attenuating their internalization and increasing their surface expression (8, 22). Here, we investigated whether Mal protein could also interact with and regulate ENaC and MLP1 in renal epithelial cells. We performed mammalian two-hybrid and dual-luciferase reporter assays using bait and prey fusion proteins to probe for putative associations between Mal, ENaC, and MLP1. pBIND-ID and pACT-MyoD fusion proteins were used as positive controls to represent maximum binding, whereas pBIND and pACT empty vectors were used as negative controls. As a second positive control, the association between γENaC and MLP1 was assessed using this assay. The assay only showed weak binding between αENaC and Mal relative to controls (Fig. 1A). Similarly, there was only a moderate association between MLP1 and Mal protein relative to controls (Fig. 1A). In order to further confirm the mammalian two-hybrid assay results, we immunoprecipitated Mal protein from cell lysates after overexpression of wild-type MLP1 or mutant MLP1 proteins in mpkCCD cells, which is constitutively membrane bound. The bound proteins were eluted and resolved by SDS-PAGE, and the blots were then probed for αENaC, βENaC, and γENaC proteins using specific antibodies. Mal protein has been shown to associate with αENaC but not with βENaC or γENaC in cells overexpressing mutant MLP1 protein but not in cells expressing wild-type MARCKS as a control (Fig. 1, B–D).
Fig. 1.
Mammalian two-hybrid dual luciferase assay probing for associations between myelin and lymphocyte (Mal), epithelial Na+ channel (ENaC), and myristoylated alanine-rich C-kinase substrate (MARCKS)-like protein 1 (MLP1). A: mouse cortical collecting duct (mpkCCDc14) cells were transfected with pG5-Luc, pACT fusion protein, and pBIND fusion protein before luciferase activities were measured from the cellular lysates. Interactions between pBIND-ID and pACT-MyoD proteins served as positive controls, whereas pBIND and pACT served as negative controls. Renilla luciferase activity served as an internal control and was used to normalize firefly luciferase activity (firefly luciferase activity divided by Renilla luciferase activity). Relative light units for the positive control group were set to 1.0. n = 3; three permeable supports of cells were analyzed for each group. B−D: immunoprecipitation (IP) Western blot (WB) analysis of αENaC (B), βENaC (C), and γENaC (D) after mpkCCD cells were transiently transfected with MARCKS wild-type (MARCKS wild) and mutant MARCKS-like protein 1 (MARCKS S3A) plasmids and Mal protein was immunoprecipitated from the cellular lysates before the bound proteins were eluted off the protein A agarose beads and resolved on SDS-PAGE gels. The blots were then probed for ENaC subunits using specific antibodies. *P < 0.05 by one-way ANOVA with the Holm-Sidak method. Ctrl, control.
Knockdown of Mal protein in wild-type mice increases ENaC open probability in native tubules.
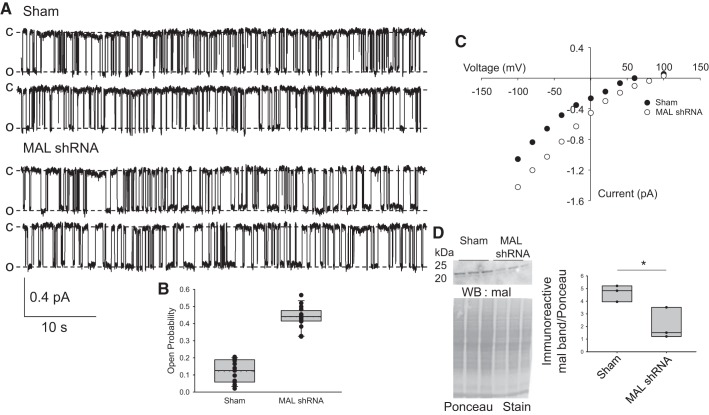
Because we observed an increase in PIP2 at the membrane of collecting duct principal cells after knockdown of Mal, we asked whether Mal protein could regulate ENaC gating in native mouse collecting duct cells. MAL shRNA lentivirus or vehicle was injected directly into 129Sv mouse kidneys, and tubules were isolated after 72 h. Collecting ducts were identified from the isolated tubules and then split open for single channel patch-clamp experiments. Single channel patch-clamp recordings revealed an increase in ENaC open probability in mice injected with MAL shRNA (0.439 ± 0.0156) compared with mice injected with vehicle alone (0.119 ± 0.0173, mean ± SE; Fig. 2).
Fig. 2.
Epithelial Na+ channel (ENaC) activity in principal cells from kidneys injected with either myelin and lymphocyte (MAL) shRNA or vehicle. A: ENaC channel activity was recorded from cell-attached patches on principal cells in collecting ducts from 129Sv mice whose kidneys had been injected with either MAL shRNA or vehicle. The top two traces are representative records from sham-injected kidneys. The bottom two traces are cells from MAL shRNA-injected kidneys. The open probability (Po) of the MAL shRNA knockdown group was substantially increased above that of the control group. Note the longer open times (MAL shRNA Po = 0.439 vs. sham Po = 0.119). Records are continuous traces filtered at 50 Hz and 57 s/trace; downward deflections are openings. All recordings were made at pipette potentials of −40 mV (difference in potential between the inside of the cell and the patch pipette; if there is a significant basal membrane potential, it will add to the pipette potential). Sham-injected data are from 15 individual patches and knockdown data are from 16 individual patches. Patches were from 7 cortical collecting ducts isolated from sham mice and 8 cortical collecting ducts from MAL shRNA-treated mice. Data are from three mice under each condition with 4, 6, and 5 patches in the sham group and 5, 4, and 7 patches in the MAL shRNA group. O, open; C, closed. B: summary of all single channel data showing that ENaC Po increased over threefold in the Mal knockdown group compared with the control group (P < 0.001 by t-test). The boxes in the plot are 25–75% confidence levels, lines are median values, and whiskers are 5% and 95% confidence levels. Dotted lines are means (very close to medians). Mean MAL shRNA Po was 0.439 ± 0.0156; the mean after sham injection was 0.119 ± 0.0173 (mean ± SE). C: current-voltage relationship for one patch on a principal cell from a sham-injected or MAL shRNA-injected kidney. The higher Po of the MAL shRNA group was presumably responsible for the slight shift of the MAL shRNA current-voltage curve to the right. D: Western blot (WB) analysis of Mal protein from the sham or MAL shRNA groups (P < 0.05 by Student’s t-test).
Mal protein expression in sucrose density gradient fractions and PLC-β3 protein association with Mal protein.
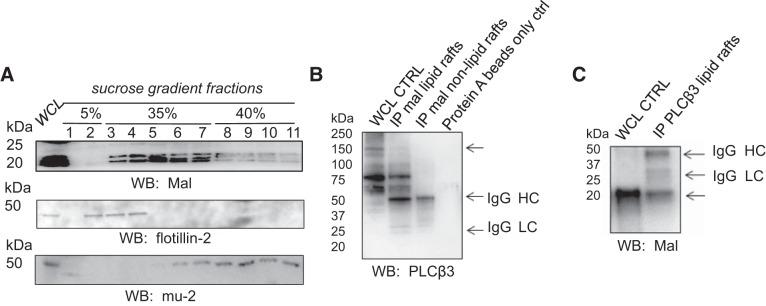
Mal protein has been shown to associate with lipid rafts in most cells (39). Here, we investigated the expression of Mal protein in mpkCCD cells. Lipid raft fractions were isolated as previously described by our group (3, 4, 40). Flotillin-2 was used as a lipid raft marker, whereas mu-2 was used as a nonlipid raft marker (Fig. 3) (13). These experiments showed, for the first time, that Mal protein was expressed in light density gradient fractions from mpkCCD cells (Fig. 3A).
Fig. 3.
Immunoprecipitation (IP) Western blot (WB) analysis showing the association between phospholipase C (PLC)-β3 protein and myelin and lymphocyte (Mal) protein in lipid raft fractions compared with nonlipid raft fractions isolated from mouse cortical collecting duct (mpkCCD) cells. A: WB of Mal protein in sucrose density gradient fractions. Flotillin-2 served as a lipid raft marker. B: IP WB after Mal protein was immunoprecipitated from lipid raft and nonlipid raft fractions. After bound proteins were eluted from the protein A beads and proteins were resolved by SDS-PAGE, the blots were probed for PLC-β3 protein expression. Omission of the primary antibody served as a negative control (CTRL). C: IP WB after PLC-β3 protein was immunoprecipitated from lipid raft fractions. PLC-β3-associated proteins were eluted from the protein A beads and resolved by SDS-PAGE before blots were probed for Mal protein using specific antibodies. HC, heavy chain of IgG; LC, light chain of IgG; WCL, whole cell lysate.
We did not observe a significant interaction between Mal and ENaC or MLP1, so we investigated putative interactions between Mal and proteins that could negatively regulate ENaC in collecting duct principal cells. PLC is known to hydrolyze PIP2 to the second messengers DAG and IP3. Hydrolysis of PIP2 would result in less available PIP2 at the membrane and consequently a decrease in ENaC open probability. Like ENaC, Mal protein was expressed in lipid raft and nonlipid raft fractions from mouse collecting duct cells (Fig. 3A). Next, we investigated a putative interaction between Mal and PLC-β3 proteins. Immunoprecipitation of Mal protein with Mal-specific antibody resulted in the pulldown of PLC-β3 in lipid raft fractions from mouse collecting duct cells (Fig. 3B). To corroborate the association between Mal protein and PLC-β3 protein, the reverse experiment was performed. Mal protein was detected by Western blot analysis in the eluant after immunoprecipitation of PLC-β3 protein with specific antibody (Fig. 3C).
Mal overexpression augments PLC-β3 expression in mpkCCD cells.
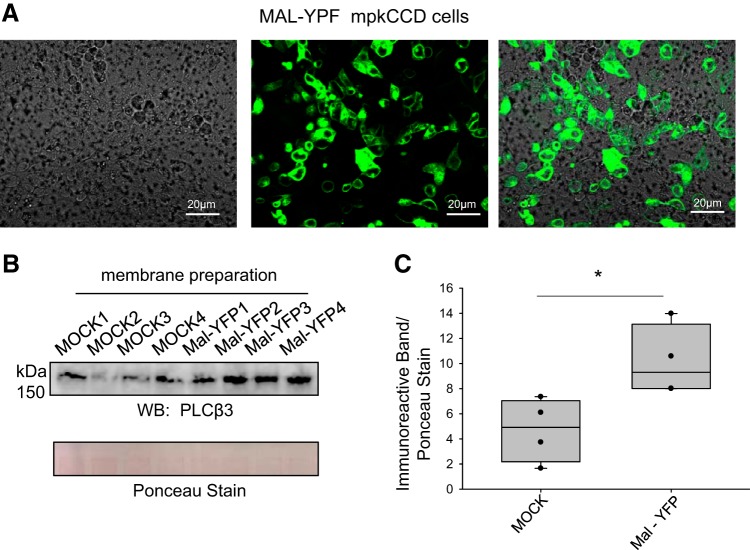
Next, we investigated whether overexpression of Mal protein could augment PLC-β3 expression in mouse collecting duct cells. mpkCCD cells were transiently transfected with MAL-YFP, and transfection was assessed by fluorescence microscopy (Fig. 4A) and Western blot analysis. Compared with the control group (4.72 ± 1.27), mpkCCD cells transfected with MAL-YFP showed an increase (10.2 ± 1.41) in PLC-β3 protein expression by Western blot analysis (P = 0.0287; Fig. 4B).
Fig. 4.
Overexpression of myelin and lymphocyte (Mal) protein augments phospholipase C (PLC)-β3 protein expression in mouse cortical collecting duct (mpkCCD) cells. A: Mal-yellow fluorescent protein (YFP) construct was transiently transfected into mpkCCD cells, and the transfection efficiency was assessed by fluorescence microscopy. Scale bars = 20 μm. B: Western blot (WB) of PLC-β3 from the particulate (membrane fraction) of mpkCCD cells transfected with either transfection reagent and buffer alone (MOCK) or MAL-YFP plasmid DNA. C: densitometric analysis of the representative WB in B. *P < 0.05 by Student’s t-test.
Mal knockdown decreases PLC-β3 protein expression in the membrane fraction.
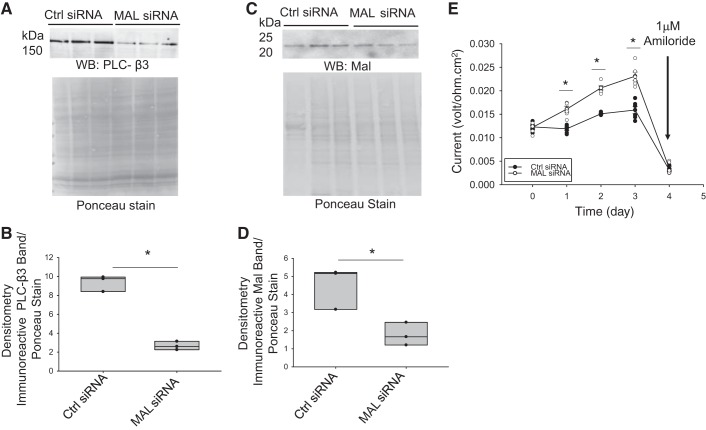
To corroborate the Mal protein overexpression experiments that suggested that Mal protein regulates PLC-β3 expression in mouse collecting duct cells, we investigated the effect of siRNA-mediated knockdown of Mal protein in mpkCCD cells on PLC-β3 expression. mpkCCD cells were transiently transfected with mouse siRNA targeting MAL when cells were 70% confluent and then again 2 days later. Cells were harvested for protein, and PLC-β3 protein expression was assessed by Western blot analysis. Compared with cells transfected with control siRNA (9.37 ± 0.487), PLC-β3 protein expression was attenuated (2.67 ± 0.260) in cells transfected with MAL siRNA (P = 0.000264; Fig. 5, A and B). Amiloride-sensitive transepithelial current increased after siRNA-mediated knockdown of MAL compared with the control group (Fig. 5E).
Fig. 5.
Phospholipase C (PLC)-β3 protein expression decreases and epithelial Na+ channel (ENaC) activity increases in mouse cortical collecting duct (mpkCCD) cells after myelin and lymphocyte (Mal) knockdown. A: representative (n = 3) Western blot (WB) for PLC-β3 after mpkCCD cells were transiently transfected with either scrambled control siRNA or siRNA targeting mouse MAL. The Ponceau-stained membrane shows total protein and served as a loading control. B: densitometric analysis of the WB in A showing that Mal knockdown dramatically reduced PLC-β3 protein expression (P < 0.05 by Student’s t-test). C: representative (n = 3) WB for Mal protein after transient transfection of mpkCCD cells with either scrambled control siRNA or MAL siRNA. D: densitometric analysis of the WB in C showing that MAL siRNA reduced Mal protein expression (P < 0.05 by Student’s t-test). E: amiloride-sensitive transepithelial current measurements from mpkCCD cells transiently transfected with either control siRNA or MAL siRNA showing that Mal knockdown increased transepithelial current (*P < 0.05 by one-way ANOVA with the Holm-Sidak method); on the last day of the measurement, we added 1 mM amiloride to show that the current was amiloride sensitive. Ctrl, control.
siRNA-mediated knockdown of MAL increases PIP2 at the plasma membrane of mpkCCD cells.
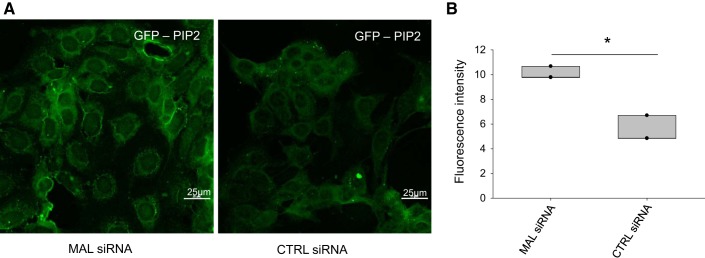
We and others have shown the anionic phosphoinositol bisphosphate PIP2 maintains ENaC in an open conformation in principal cells of the collecting duct (3, 4, 30). MARCKS and members of the MARCKS family, including MLP1, play an essential role in serving as an adaptor protein between PIP2 and ENaC. Here, we investigated whether Mal protein could regulate PIP2 in the plasma membrane. Since other groups have shown PIP2 is localized to the apical plasma membrane of mammalian kidney cells (42, 48), PIP2 itself can be used as a cell surface marker. We performed knockdown experiments of MAL in mpkCCD cells using MAL-specific siRNA. Compared with control scrambled siRNA (5.465 ± 0.618), mpkCCD cells transfected with MAL siRNA showed an increase (10.1 ± 0.298) in PIP2 at the plasma membrane as measured by florescence microscopy using the PIP2 reporter GFP-PLC-δ pleckstrin homology domain (P = 0.00127; Fig. 6).
Fig. 6.
siRNA-mediated knockdown of myelin and lymphocyte (MAL) in mouse cortical collecting duct (mpkCCD) cells results in phosphatidylinositol 4,5-bisphosphate (PIP2) accumulation at the luminal membrane of mpkCCD cells. A: representative (n = 3) immunofluorescence images of the PIP2 reporter green fluorescent protein (GFP)-phospholipase C-δ pleckstrin homology domain after mpkCCD cells were transfected with either scrambled or MAL siRNA. The PIP2 reporter was more prominent after MAL siRNA-mediated knockdown. B: fluorescence intensity analysis of the images in A showing the reduction in PIP2 reporter. Images were taken using a Nikon fluorescence microscope at a magnification of ×60. *P < 0.05 by Student’s t-test. CTRL, control.
Mal protein is attenuated in salt-loaded 129Sv wild-type mice.
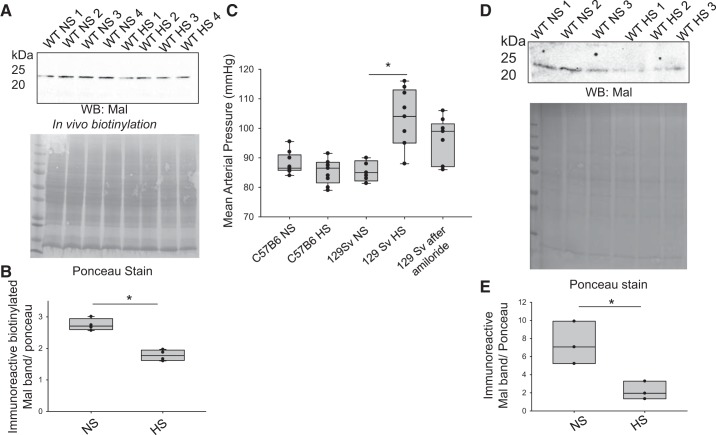
Salt loading of 129Sv mice results in a transient increase in mean arterial blood pressure. Since the increase of renal ENaC activity contributes to salt-sensitive hypertension and we showed here that Mal protein negatively regulates ENaC, we also investigated Mal protein expression in the kidneys of salt-loaded and control mice. Mouse kidneys were subject to in vivo biotinylation as previously described by Bao et al. (6) to label membrane proteins, and the amount of membrane-bound Mal protein was examined by Western blot analysis. Mal protein expression at the luminal membrane in the kidney cortex of salt-loaded 129Sv mice was less (1,780 ± 86.2) compared with non-salt-loaded mice (2,750 ± 94.5, P = 0.000136; Fig. 7, A and B). Salt-loaded 129Sv mice showed higher mean arterial blood pressure compared with non-salt-loaded 129Sv mice, but there was no difference in blood pressure before and after salt loading in the salt-insensitive C57B6 mice (Fig. 7C). Mal protein decreased in kidney cortex membrane fractions (Fig. 7, D and E), as seen in biotinylated fractions.
Fig. 7.
Myelin and lymphocyte (Mal) protein expression is attenuated in the kidneys of salt-loaded 129Sv mice. A: Western blot (WB) of Mal protein in the streptavidin precipitate from in situ biotinylated 129Sv wild-type (WT) mouse kidney cortex tissue lysate (100 μg total protein) after mice were maintained on either a normal-salt (NS) or high-salt (HS) diet for 2 wk (n = 4 for each group). B: densitometric analysis of the WB in A normalized to the Ponceau-stained membrane. *P < 0.05. C: tail-cuff blood pressure measurements of a cohort of 129Sv mice and C57B6 mice before and after salt loading for 2 wk, showing that 129Sv mice have a strong increase in blood pressure after salt loading, whereas C57B6 mice do not respond to salt loading. Only the 129Sv group received amiloride, an epithelial Na+ channel blocker, at the end of the study (n = 3 for each group). D: WB of Mal protein in kidney cortex membrane fractions from 129Sv mice maintained on a NS or HS diet and the corresponding Ponceau stain. E: densitometric analysis of the WB in D showing that Mal protein decreased in kidney cortex membrane fractions as it did in biotinylated fractions. *P < 0.05 by Student’s t-test.
DISCUSSION
The physiological significance of Mal protein in the kidney is underscored by this protein regulating ENaC, a known regulator of total body water and Na+ transport (19). Although there are two proteins in the Mal family, here we focused on Mal protein because Mal2 has been previously shown not to form complexes with apical proteins (39).
Renal ENaC protein expression and activity in the luminal plasma membrane are regulated by a myriad of proteins that indirectly or directly interact with the channel (2, 3, 21, 41, 50, 58). These proteins alter the density of ENaC at the membrane and/or the open probability of the channel. For example, the ubiquitin ligase neural precursor cell-expressed developmentally downregulated 4-2 (Nedd4-2) binds PY motifs in the COOH-termini of membrane-bound ENaC subunits, resulting in a decrease in the density of ENaC surface expression (58). Aldosterone activates the serum and glucocorticoid regulated kinase, which phosphorylates Nedd4-2, leading to the binding between 14-3-3 proteins and Nedd4-2 (21). The interaction between 14-3-3 protein and Nedd4-2 prevents the association between Nedd4-2 and ENaC and thus prevents degradation of the channel (51).
Here, we investigated whether Mal associates with renal ENaC and proteins, such as MARCKS and MLP1, that bind anionic phospholipid phosphates and regulate ENaC at the apical membrane of distal tubule and collecting duct cells. MARCKS and MLP1 sequester the signaling lipid PIP2 at the apical plasma membrane of distal tubule and collecting duct cells to stabilize ENaC in an open confirmation (3). Although the MARCKS family of proteins serves as an adaptor protein between PIP2 and ENaC, PLC hydrolyzes PIP2 to DAG and IP3, thereby reducing the interaction between PIP2 and ENaC and consequently decreasing the probability of ENaC being in an open state.
Mal protein is known to regulate protein expression in the kidney by either stabilizing proteins at the apical membrane, as it does with NKCC2 (8), or by increasing the steady-state phosphorylation of the protein, as it does with AQP2 (22). Therefore, we explored multiple mechanisms by which Mal could negatively regulate ENaC. One possible mechanism involves Mal protein competitively binding to proteins that stabilize ENaC. However, we did not observe any appreciable binding between Mal and any members of the MARCKS family. Another possible mechanism involves Mal stabilizing a protein that could potentially negatively regulate ENaC. PKC is one such protein that phosphorylates MARCKS and renders it inactive in the cytoplasm (16). However, we did not observe an association between Mal and PKC (data not shown). PLC is another protein that can negatively regulate ENaC, as it hydrolyzes PIP2 and decrease its availability to bind ENaC and maintain it in an open confirmation. Knockdown and overexpression experiments were performed to establish a role for Mal protein in the negative regulation of renal ENaC.
To our knowledge, Mal and PLC-β3 protein expression in lipid raft-enriched fractions from mouse principal cells has not been reported. We have previously demonstrated that a subpopulation of ENaC and MARCKS are enriched in light density gradient sucrose fractions (3). Lipid rafts are thought to function as signaling microdomains and serve as an organizing center for stabilizing protein complexes (35, 49). However, in the context of PIP2 regulation at the luminal plasma membrane of principal cells, Mal protein may serve as a means of regulating the availability of PIP2 in close proximity to ENaC through the recruitment of PLC-β3. The results from the experiments presented here suggest that a complex but precise feedback mechanism exists for the regulation of ENaC by PIP2. This mechanism involves the MARCKS family of proteins sequestering PIP2 and increasing its local concentration at the luminal membrane to positively regulate the gating of ENaC, while Mal hydrolyzes PIP2 and decreases its availability to negatively regulate ENaC gating.
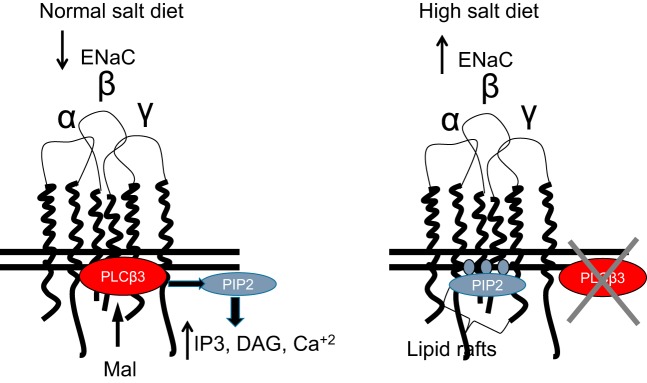
An upregulation of ENaC expression and activity is known to contribute to the development of salt-sensitive hypertension (34, 38, 44). Therefore, we investigated the effect of salt loading of 129Sv wild-type mice on Mal protein expression. Figure 8 shows a schematic diagram of our proposed model. With a normal-salt diet, Mal protein is expressed and stabilizes PLC-β3; PLC activity reduces available PIP2 in the membrane (producing the PIP2 degradation products IP3 and DAG and increasing intracellular Ca2+). Depletion of PIP2 in the luminal membrane reduces ENaC activity by reducing the mean open time. On a high-salt diet, Mal no longer stabilizes PLC-β3 in apical lipid rafts, leading to increased PIP2 in the luminal membrane of collecting duct cells; this results in the stabilization of ENaC in an open conformation (Fig. 8).
Fig. 8.
Schematic diagram depicting the effects of myelin and lymphocyte (Mal) protein on phospholipase C (PLC)-β3 protein expression and epithelial Na+ channel (ENaC) activity in mouse collecting duct cells. Mal protein stabilizes PLC-β3 protein expression in lipid rafts of collecting duct cells from salt-sensitive wild-type mice maintained on a normal-salt diet. This results in low phosphatidylinositol 4,5-bisphosphate (PIP2) abundance at the luminal plasma membrane and normal basal ENaC activity that includes the channel being either in the open or closed state. Salt loading of 129Sv wild-type mice results in less Mal and less PLC-β3 protein expression at the luminal plasma membrane and an increase in PIP2 abundance and presumably its association with ENaC. This results in ENaC being locked in an open position and present in a constitutively active state. PLC-β3 has been shown to hydrolyze PIP2 to generate the second messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), and IP3 subsequently results in Ca2+ release by activating channels in the endoplasmic reticulum/sarcoplasmic reticulum.
The physiological significance of Mal in principal cells of the collecting duct is exemplified by its role in the organization and stabilization of lipid rafts and regulation of ENaC activity and effector proteins that regulate the association of ENaC expression and gating at the apical plasma membrane. Since Mal protein regulates AQP channels and multiple Na+ transport mechanisms in the kidney, future whole animal studies are needed to determine whether Mal serves as a negative feedback regulator to maintain total body water and Na+ homeostasis. Also, additional studies are needed to determine whether Mal plays a role in the regulation of long-term blood pressure.
GRANTS
This work was funded in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant K01-DK-099617 (to A. A. Alli) and the University of Florida College of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.T., D.C.E., and A.A.A. conceived and designed research; K.M.T., B.C.L., Q.Y., Z.M.G., and A.A.A. performed experiments; K.M.T., B.C.L., Q.Y., Z.M.G., and A.A.A. analyzed data; K.M.T., D.C.E., and A.A.A. interpreted results of experiments; K.M.T., Q.Y., D.C.E., and A.A.A. prepared figures; A.A.A. drafted manuscript; K.M.T., Z.M.G., H.P.M., D.C.E., and A.A.A. edited and revised manuscript; K.M.T., B.C.L., Q.Y., Z.M.G., H.P.M., D.C.E., and A.A.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Otor Al-Khalili for preparing plasmids that were used to perform transient transfections. Fluorescent images were taken using a Nikon A1RMP confocal microscope at the University of Florida’s Cell and Tissue Analysis Core (National Institutes of Health Grant 1-S10-OD-020026).
REFERENCES
- 1.Aguilar RC, Ohno H, Roche KW, Bonifacino JS. Functional domain mapping of the clathrin-associated adaptor medium chains mu1 and mu2. J Biol Chem 272: 27160–27166, 1997. doi: 10.1074/jbc.272.43.27160. [DOI] [PubMed] [Google Scholar]
- 2.Alli A, Yu L, Holzworth M, Richards J, Cheng KY, Lynch IJ, Wingo CS, Gumz ML. Direct and indirect inhibition of the circadian clock protein Per1: effects on ENaC and blood pressure. Am J Physiol Renal Physiol 316: F807–F813, 2019. doi: 10.1152/ajprenal.00408.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alli AA, Bao HF, Alli AA, Aldrugh Y, Song JZ, Ma HP, Yu L, Al-Khalili O, Eaton DC. Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am J Physiol Renal Physiol 303: F800–F811, 2012. doi: 10.1152/ajprenal.00703.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alli AA, Bao HF, Liu BC, Yu L, Aldrugh S, Montgomery DS, Ma HP, Eaton DC. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am J Physiol Renal Physiol 309: F456–F463, 2015. doi: 10.1152/ajprenal.00631.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alli AA, Song JZ, Al-Khalili O, Bao HF, Ma HP, Alli AA, Eaton DC. Cathepsin B is secreted apically from Xenopus 2F3 cells and cleaves the epithelial sodium channel (ENaC) to increase its activity. J Biol Chem 287: 30073–30083, 2012. doi: 10.1074/jbc.M111.338574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao HF, Thai TL, Yue Q, Ma HP, Eaton AF, Cai H, Klein JD, Sands JM, Eaton DC. ENaC activity is increased in isolated, split-open cortical collecting ducts from protein kinase Cα knockout mice. Am J Physiol Renal Physiol 306: F309–F320, 2014. doi: 10.1152/ajprenal.00519.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao HF, Zhang ZR, Liang YY, Ma JJ, Eaton DC, Ma HP. Ceramide mediates inhibition of the renal epithelial sodium channel by tumor necrosis factor-α through protein kinase C. Am J Physiol Renal Physiol 293: F1178–F1186, 2007. doi: 10.1152/ajprenal.00153.2007. [DOI] [PubMed] [Google Scholar]
- 8.Carmosino M, Rizzo F, Procino G, Basco D, Valenti G, Forbush B, Schaeren-Wiemers N, Caplan MJ, Svelto M. MAL/VIP17, a new player in the regulation of NKCC2 in the kidney. Mol Biol Cell 21: 3985–3997, 2010. doi: 10.1091/mbc.e10-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cha SH, Cha JH, Cho YJ, Noh DY, Lee KH, Endou H. Distributional patterns of phospholipase C isozymes in rat kidney. Nephron 80: 314–323, 1998. doi: 10.1159/000045192. [DOI] [PubMed] [Google Scholar]
- 10.Cheong KH, Zacchetti D, Schneeberger EE, Simons K. VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells. Proc Natl Acad Sci USA 96: 6241–6248, 1999. doi: 10.1073/pnas.96.11.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Marco MC, Martín-Belmonte F, Kremer L, Albar JP, Correas I, Vaerman JP, Marazuela M, Byrne JA, Alonso MA. MAL2, a novel raft protein of the MAL family, is an essential component of the machinery for transcytosis in hepatoma HepG2 cells. J Cell Biol 159: 37–44, 2002. doi: 10.1083/jcb.200206033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espana-Agusti J, Tuveson DA, Adams DJ, Matakidou A. A minimally invasive, lentiviral based method for the rapid and sustained genetic manipulation of renal tubules. Sci Rep 5: 11061, 2015. doi: 10.1038/srep11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng X, Huang H, Yang Y, Fröhlich O, Klein JD, Sands JM, Chen G. Caveolin-1 directly interacts with UT-A1 urea transporter: the role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane. Am J Physiol Renal Physiol 296: F1514–F1520, 2009. doi: 10.1152/ajprenal.00068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank M. MAL, a proteolipid in glycosphingolipid enriched domains: functional implications in myelin and beyond. Prog Neurobiol 60: 531–544, 2000. doi: 10.1016/S0301-0082(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 15.Frank M, van der Haar ME, Schaeren-Wiemers N, Schwab ME. rMAL is a glycosphingolipid-associated protein of myelin and apical membranes of epithelial cells in kidney and stomach. J Neurosci 18: 4901–4913, 1998. doi: 10.1523/JNEUROSCI.18-13-04901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadi D, Wagenknecht-Wiesner A, Holowka D, Baird B. Sequestration of phosphoinositides by mutated MARCKS effector domain inhibits stimulated Ca2+ mobilization and degranulation in mast cells. Mol Biol Cell 22: 4908–4917, 2011. doi: 10.1091/mbc.e11-07-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Caballero A, Dang Y, He H, Stutts MJ. ENaC proteolytic regulation by channel-activating protease 2. J Gen Physiol 132: 521–535, 2008. doi: 10.1085/jgp.200810030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haerteis S, Krappitz M, Bertog M, Krappitz A, Baraznenok V, Henderson I, Lindström E, Murphy JE, Bunnett NW, Korbmacher C. Proteolytic activation of the epithelial sodium channel (ENaC) by the cysteine protease cathepsin-S. Pflugers Arch 464: 353–365, 2012. doi: 10.1007/s00424-012-1138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 579: 95–132, 2016. doi: 10.1016/j.gene.2015.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 21.Ichimura T, Yamamura H, Sasamoto K, Tominaga Y, Taoka M, Kakiuchi K, Shinkawa T, Takahashi N, Shimada S, Isobe T. 14-3-3 proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J Biol Chem 280: 13187–13194, 2005. doi: 10.1074/jbc.M412884200. [DOI] [PubMed] [Google Scholar]
- 22.Kamsteeg EJ, Duffield AS, Konings IB, Spencer J, Pagel P, Deen PM, Caplan MJ. MAL decreases the internalization of the aquaporin-2 water channel. Proc Natl Acad Sci USA 104: 16696–16701, 2007. doi: 10.1073/pnas.0708023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma HP, Al-Khalili O, Ramosevac S, Saxena S, Liang YY, Warnock DG, Eaton DC. Steroids and exogenous gamma-ENaC subunit modulate cation channels formed by alpha-ENaC in human B lymphocytes. J Biol Chem 279: 33206–33212, 2004. doi: 10.1074/jbc.M405455200. [DOI] [PubMed] [Google Scholar]
- 24.Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflugers Arch 455: 169–180, 2007. doi: 10.1007/s00424-007-0294-3. [DOI] [PubMed] [Google Scholar]
- 25.Ma HP, Eaton DC. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol 16: 3182–3187, 2005. doi: 10.1681/ASN.2005040434. [DOI] [PubMed] [Google Scholar]
- 26.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed expressed epithelial sodium channel (ENaC). J Biol Chem 277: 7641–7644, 2002. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- 27.Magyar JP, Ebensperger C, Schaeren-Wiemers N, Suter U. Myelin and lymphocyte protein (MAL/MVP17/VIP17) and plasmolipin are members of an extended gene family. Gene 189: 269–275, 1997. doi: 10.1016/S0378-1119(96)00861-X. [DOI] [PubMed] [Google Scholar]
- 28.Malik B, Schlanger L, Al-Khalili O, Bao HF, Yue G, Price SR, Mitch WE, Eaton DC. Enac degradation in A6 cells by the ubiquitin-proteosome proteolytic pathway. J Biol Chem 276: 12903–12910, 2001. doi: 10.1074/jbc.M010626200. [DOI] [PubMed] [Google Scholar]
- 29.Mazuruk K, Schoen TJ, Chader GJ, Rodriguez IR. Structural organization and expression of the human phosphatidylinositol-specific phospholipase C beta-3 gene. Biochem Biophys Res Commun 212: 190–195, 1995. doi: 10.1006/bbrc.1995.1955. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery DS, Yu L, Ghazi ZM, Thai TL, Al-Khalili O, Ma HP, Eaton DC, Alli AA. ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins. Am J Physiol Cell Physiol 313: C42–C53, 2017. doi: 10.1152/ajpcell.00244.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura A, Imaizumi A, Yanagawa Y. [Beta 2-adrenoceptor function in the kidney]. Nihon Yakurigaku Zasshi 124: 427–434, 2004. doi: 10.1254/fpj.124.427. [DOI] [PubMed] [Google Scholar]
- 32.Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem 283: 36586–36591, 2008. doi: 10.1074/jbc.M805676200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel AB, Chao J, Palmer LG. Tissue kallikrein activation of the epithelial Na channel. Am J Physiol Renal Physiol 303: F540–F550, 2012. doi: 10.1152/ajprenal.00133.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlov TS, Staruschenko A. Involvement of ENaC in the development of salt-sensitive hypertension. Am J Physiol Renal Physiol 313: F135–F140, 2017. doi: 10.1152/ajprenal.00427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J 378: 281–292, 2004. doi: 10.1042/bj20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pochynyuk O, Bugaj V, Stockand JD. Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr Opin Nephrol Hypertens 17: 533–540, 2008. doi: 10.1097/MNH.0b013e328308fff3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pochynyuk O, Tong Q, Staruschenko A, Ma HP, Stockand JD. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am J Physiol Renal Physiol 290: F949–F957, 2006. doi: 10.1152/ajprenal.00386.2005. [DOI] [PubMed] [Google Scholar]
- 38.Pratt JH. Central role for ENaC in development of hypertension. J Am Soc Nephrol 16: 3154–3159, 2005. doi: 10.1681/ASN.2005050460. [DOI] [PubMed] [Google Scholar]
- 39.Ramnarayanan SP, Tuma PL. MAL, but not MAL2, expression promotes the formation of cholesterol-dependent membrane domains that recruit apical proteins. Biochem J 439: 497–504, 2011. doi: 10.1042/BJ20110803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reifenberger MS, Yu L, Bao HF, Duke BJ, Liu BC, Ma HP, Alli AA, Eaton DC, Alli AA. Cytochalasin E alters the cytoskeleton and decreases ENaC activity in Xenopus 2F3 cells. Am J Physiol Renal Physiol 307: F86–F95, 2014. doi: 10.1152/ajprenal.00251.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rotin D, Schild L. ENaC and its regulatory proteins as drug targets for blood pressure control. Curr Drug Targets 9: 709–716, 2008. doi: 10.2174/138945008785132367. [DOI] [PubMed] [Google Scholar]
- 42.Skwarek LC, Boulianne GL. Great expectations for PIP: phosphoinositides as regulators of signaling during development and disease. Dev Cell 16: 12–20, 2009. doi: 10.1016/j.devcel.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Stockand JD, Bao HF, Schenck J, Malik B, Middleton P, Schlanger LE, Eaton DC. Differential effects of protein kinase C on the levels of epithelial Na+ channel subunit proteins. J Biol Chem 275: 25760–25765, 2000. doi: 10.1074/jbc.M003615200. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y, Zhang JN, Zhao D, Wang QS, Gu YC, Ma HP, Zhang ZR. Role of the epithelial sodium channel in salt-sensitive hypertension. Acta Pharmacol Sin 32: 789–797, 2011. doi: 10.1038/aps.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takiar V, Mistry K, Carmosino M, Schaeren-Wiemers N, Caplan MJ. VIP17/MAL expression modulates epithelial cyst formation and ciliogenesis. Am J Physiol Cell Physiol 303: C862–C871, 2012. doi: 10.1152/ajpcell.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607–610, 1997. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 47.Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol 120: 191–201, 2002. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Richards DA. Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane. Biol Open 1: 857–862, 2012. doi: 10.1242/bio.20122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang XQ, Paller AS. Lipid rafts: membrane triage centers. J Invest Dermatol 126: 951–953, 2006. doi: 10.1038/sj.jid.5700282. [DOI] [PubMed] [Google Scholar]
- 50.Ware AW, Cheung TT, Rasulov S, Burstein E, McDonald FJ. Epithelial Na+ channel: reciprocal control by COMMD10 and Nedd4-2. Front Physiol 9: 793, 2018. doi: 10.3389/fphys.2018.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang B, Kumar S. Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ 17: 68–77, 2010. doi: 10.1038/cdd.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu L, Al-Khalili O, Duke BJ, Stockand JD, Eaton DC, Bao HF. The inhibitory effect of Gβγ and Gβ isoform specificity on ENaC activity. Am J Physiol Renal Physiol 305: F1365–F1373, 2013. doi: 10.1152/ajprenal.00009.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu L, Eaton DC, Helms MN. Effect of divalent heavy metals on epithelial Na+ channels in A6 cells. Am J Physiol Renal Physiol 293: F236–F244, 2007. doi: 10.1152/ajprenal.00002.2007. [DOI] [PubMed] [Google Scholar]
- 54.Yue G, Edinger RS, Bao HF, Johnson JP, Eaton DC. The effect of rapamycin on single ENaC channel activity and phosphorylation in A6 cells. Am J Physiol Cell Physiol 279: C81–C88, 2000. doi: 10.1152/ajpcell.2000.279.1.C81. [DOI] [PubMed] [Google Scholar]
- 55.Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969, 2002. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- 56.Zacchetti D, Peränen J, Murata M, Fiedler K, Simons K. VIP17/MAL, a proteolipid in apical transport vesicles. FEBS Lett 377: 465–469, 1995. doi: 10.1016/0014-5793(95)01396-2. [DOI] [PubMed] [Google Scholar]
- 57.Zhang ZR, Chou CF, Wang J, Liang YY, Ma HP. Anionic phospholipids differentially regulate the epithelial sodium channel (ENaC) by interacting with alpha, beta, and gamma ENaC subunits. Pflugers Arch 459: 377–387, 2010. doi: 10.1007/s00424-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou R, Patel SV, Snyder PM. Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem 282: 20207–20212, 2007. doi: 10.1074/jbc.M611329200. [DOI] [PubMed] [Google Scholar]