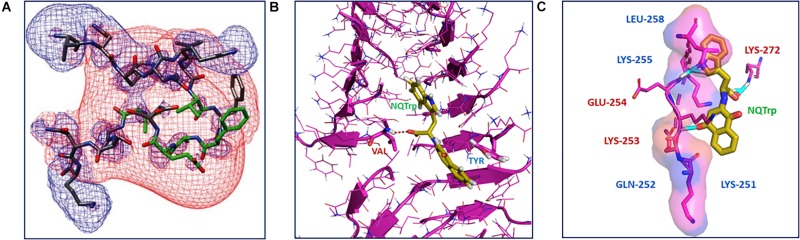
FIGURE 2.
Interaction of NQTrp with amyloidogenic peptides: (A) NMR spectroscopy analysis of Aβ peptide with NQTrp. Lowest energy structure generated for Aβ12–28 with NQTrp (Molar ratio 4:1), where the residues colored in green showed significant deviations upon binding NQTrp. The negative (red) and positive (blue) electrostatic potential distribution for ±2 kT/e are mapped onto the structure (Image reproduced from Scherzer-Attali et al., 2010). (B) Complexation of NQTrp with Tau-derived PHF6 peptides in the fibrillar arrangement during disassembly, observed during molecular dynamics simulation (Image reproduced from KrishnaKumar et al., 2018b). (C) Putative interaction sites of PAPf39 peptide with NQTrp leading to the inhibiton of its agregation, visualized using molecular docking (Image reproduced from Viswanathan et al., 2018). Interacting residues are shown as sticks.