Abstract
Citrus sinensis seedlings were irrigated with nutrient solution at a concentration of 0 (Mg-deficiency) or 2 (Mg-sufficiency) mM Mg (NO3)2 for 16 weeks. Mg-deficiency-induced interveinal chlorosis, vein enlargement and corkiness, and alterations of gas exchange, pigments, chlorophyll a fluorescence (OJIP) transients and related parameters were observed in middle and lower leaves, especially in the latter, but not in upper leaves. Mg-deficiency might impair the whole photosynthetic electron transport, including structural damage to thylakoids, ungrouping of photosystem II (PSII), inactivation of oxygen-evolving complex (OEC) and reaction centers (RCs), increased reduction of primary quinone electron acceptor (QA) and plastoquinone pool at PSII acceptor side and oxidation of PSI end-electron acceptors, thus lowering energy transfer and absorption efficiency and the transfer of electrons to the dark reactions, hence, the rate of CO2 assimilation in Mg-deficiency middle and lower leaves. Although potassium, Mg, manganese and zinc concentration in blades displayed a significant and positive relationship with the corresponding element concentration in veins, respectively, great differences existed in Mg-deficiency-induced alterations of nutrient concentrations between leaf blades and veins. For example, Mg-deficiency increased boron level in the blades of upper leaves, decreased boron level in the blades of lower leaves, but did not affect boron level in the blades of middle leaves and veins of upper, middle and lower leaves. To conclude, Mg-deficiency-induced interveinal chlorosis, vein enlargement, and corkiness, and alterations to photosynthesis and related parameters increased with increasing leaf age. Mg-deficiency-induced enlargement and corkiness of veins were not caused by Mg-deficiency-induced boron-starvation.
Keywords: Citrus sinensis, leaf blade, magnesium (Mg)-deficiency, OJIP transient, photosynthesis, vein enlargement and corkiness
1. Introduction
Magnesium (Mg), as a central element of the chlorophyll (Chl) molecule and the activator of more than 300 enzymes, plays key roles in various physiological and biochemical processes, including Chl biosynthesis and photosynthesis [1,2,3,4,5]. Despite its vital roles in the normal growth and development of higher plants, less attention has been paid to Mg in comparison to nitrogen (N), phosphorus (P), and potassium (K) [6]. Mg-deficiency, one of the most common physiological disorder influencing productivity and quality of crops including Citrus, is being intensified in intensive agricultural systems, where N, P, and K fertilizers have been heavily applied [7,8,9,10,11].
In addition to interveinal chlorosis, the decrease in leaf CO2 assimilation in response to Mg-deficiency is often observed in many higher plants, including Citrus [10,12,13,14,15,16]. As the transport of Mg from the old leaves to the young leaves is elevated under Mg-limited conditions, Mg-deficiency symptoms first appear in old leaves, and progressively extend toward the young leaves as the exposure duration of Mg-deficiency prolongs [1,3,8]. This leads us to hypothesize that Mg-deficiency-induced decreases in leaf pigments and photosynthesis, and alterations to Chl, fluorescence (OJIP) transients, and related fluorescence parameters might become more pronounced with increasing leaf age. To our knowledge, such data are very limited. Moreover, the results are not consistent. Li et al. and Cai et al. observed that CO2 assimilation, stomatal conductance (gs), and Chl a, Chl b, Chl a + b, and carotenoid (Car) concentrations were greatly decreased in Mg-deficiency lower leaves of Citrus sinensis seedlings, but unaltered in Mg-deficiency upper leaves except for a slight decrease in CO2 assimilation [8,11]. Although a few, very small necrotic spots occurred in source leaves of Mg-deficiency spinach leaves, Chl concentration in Mg-deficiency source and sink leaves remained unaltered relative to controls [17]. Kobayashi et al. investigated the effects of 6 and 8 days of Mg-deficiency on Chl, transpiration rate, and photosynthesis in leaf 4, leaf 5, and leaf 6 blades (from bottom to top) of rice seedlings. Chl level was reduced only in leaf 5 and leaf 6 on day 8. Transpiration in leaf 5 was 55% and 26% lower than that in controls on days 6 and 8, respectively [18]. Photosynthesis was decreased only in leaf 5 on day 8.
Monitoring OJIP has been widely used to examine the effects of nutrients (viz. Mg, N, P, K, calcium (Ca), sulfur (S), boron (B), iron (Fe), and manganese (Mn)) deficiencies on the behavior of the photosystem II (PSII) [19,20,21,22,23,24,25,26,27], since OJIP is a nonmultilative, super-sensitive, reliable, rapidly and simply measured approach, and it can provide a great deal of significant data about the photosynthetic apparatus [28,29,30,31]. However, very limited data are available on the effects of nutrient deficiencies on OJIP transients and related fluorescence parameters in different age (positioned) leaves. In a study with winter wheat, Živčák et al. found that N-deficiency lowered maximum PSII efficiency of dark-adapted leaves (Fv/Fm), performance index (PIabs), and total performance index (PIabs,total) in the first, youngest leaf (leaf 1) and the third leaf (leaf 3), especially in leaf 3 with the only exception that Fv/Fm in the leaf 1 was not decreased by N deficiency [32]. Recently, Cai et al. observed Mg-deficiency-induced decreases in Fv/Fm, fraction of oxygen-evolving complex (OEC) in comparison with control and PIabs,total, and increases in quantum yield for energy dissipation (DIo/ABS) and dissipated energy flux per reaction center (RC, DIo/RC) were greater in lower leaves than those in upper leaves in C. sinensis seedlings [8]. Unfortunately, OJIP transients and many other fluorescence parameters were not provided in the two studies.
Magnesium-deficiency affects the uptake of nutrients and their concentrations in roots, stems, and leaves [1,2,33,34]. Evidence shows that Mg-deficiency may improve the concentrations of other cations, such as Ca, K, and Mn [2,11,35,36]. To our knowledge, very little is known about Mg-deficiency effects on concentrations of nutrients in various positioned (age) leaves. In a study with maize, Jezek et al. observed that Mg-deficiency increased the concentrations of K and Mg in the leaf 6, leaf 7, leaf 8, and the youngest leaf, and Mg-deficiency-induced alteration of K concentration was greater in the leaf 6 and leaf 7 than those in the leaf 8 and youngest leaf, while Mg-deficiency-induced alteration of Mn concentration was the lowest in the leaf 6 [2].
Leaf vein networks play key roles in transporting nutrition effectively into leaf cells [37,38]. The concentrations of some elements were higher in leaf veins than those in blades [38,39]. Thus, nutrient-deficiency-induced alterations of element levels might be different between leaf vein and blades. So far, such data have not been reported.
Like boron (B)-deficiency symptoms [40], enlargement and corkiness of midribs and main lateral veins often occur in Mg-deficiency Citrus leaves [8,12,33]. However, it is not clear whether the symptoms in Mg-deficiency leaves are caused by Mg-deficiency-induced B-starvation or not. So far, such symptoms were not found in other higher plants.
Here, we used C. sinensis seedlings as materials, and investigated Mg-deficiency symptoms and effects on pigments, gas exchange, OJIP transients, and related fluorescence parameters in upper, middle, and lower leaves, and concentrations of Mg and other elements in the blades (midribs, main lateral veins, and petioles removed) and veins of upper, middle, and lower leaves. The objectives were (a) to corroborate the hypotheses that Mg-deficiency-induced interveinal chlorosis, vein enlargement and corkiness, and alterations of photosynthesis and related physiological parameters increase with increasing leaf age and that Mg-deficiency-induced alterations to element levels differ between leaf blades and veins, and (b) to determine whether Mg-deficiency-induced enlargement and corkiness of veins are caused by Mg-deficiency-induced B-starvation.
2. Results
2.1. Mg-Deficiency Symptoms in C. sinensis Seedlings
As shown in Figure 1, 0 mM Mg treatment markedly inhibited root and shoot growth. Mg-deficiency symptoms first appeared in the basal older leaves, and then gradually extended toward the top young leaves. Interveinal chlorosis was observed in 0 mM Mg-treated middle and lower leaves, but not in 0 mM Mg-treated upper leaves. Enlargement and corkiness of midribs and main lateral veins were observed in a large number of 0 mM Mg-treated lower leaves and a small number of 0 mM Mg-treated middle leaves. No such Mg-deficiency symptoms occurred in 2 mM Mg-treated upper, middle, and lower leaves. Thus, seedlings that received 2 mM and 0 mM Mg were considered as Mg-sufficiency (control) and Mg-deficiency, respectively.
Figure 1.
Mg-deficiency symptoms on Citrus sinensis seedlings.
2.2. Leaf Pigments and Gas Exchange
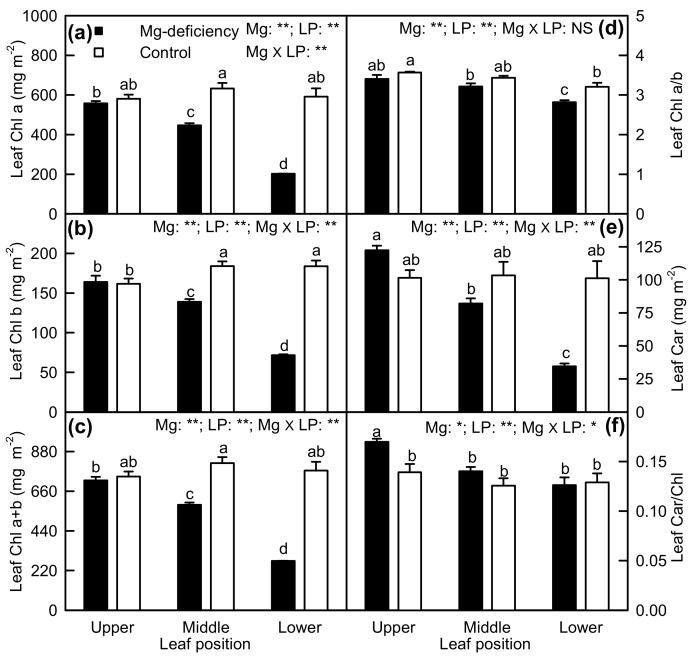
As shown in Figure 2, Mg-deficiency lowered Chl a, Chl b, Chl a + b, and Car concentrations in middle and lower leaves, but had no influence on their concentrations in upper leaves. Mg-deficiency decreased the Chl a/b ratio in lower leaves and increased the Car/Chl ratio in upper leaves. Under Mg-deficiency, Chl a, Chl b, Chl a + b, and Car concentrations decreased with increasing leaf age, but under Mg-sufficiency, their concentrations did not vary with leaf positions except for a slightly decreased Chl a concentration in upper leaves. The Chl a/b ratio slightly decreased with increasing leaf age, but the Car/Chl ratio was similar among the six treatment combinations except for a significant increase in Mg-deficiency upper leaves. There were significant interactions between Mg and leaf positions for the six parameters except for Chl a/b .
Figure 2.
Mg-deficiency effects on Chl a (a), Chl b (b), Chl a + b (c), Chl a/b ratio (d), Car (e) and Car/Chl ratio (f) in C. sinensis leaves. Bars represent mean ± standard error (n = 4). Data were analyzed by two-way ANOVA (three (leaf positions) × two (Mg levels)) followed by Duncan's multiple range. Different letters above the bars indicate a significant difference at p < 0.05. NS, *, and ** indicate nonsignificant, significant at 5% and 1% level, respectively. LP, leaf position.
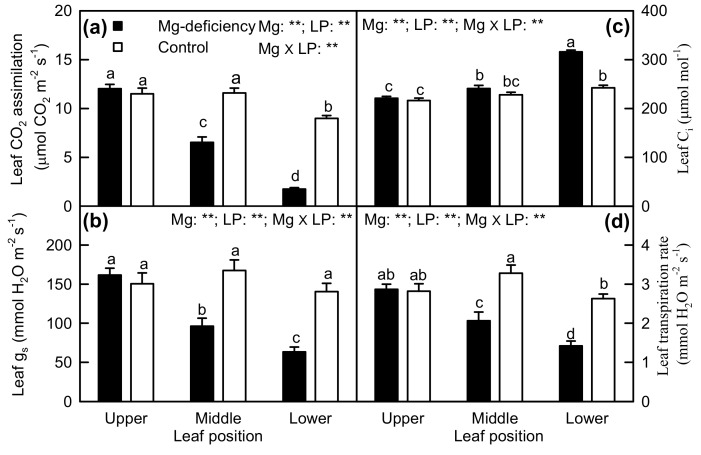
Magnesium-deficiency decreased CO2 assimilation, gs, and transpiration rate in middle and lower leaves and increased intercellular CO2 concentration (Ci) in lower leaves, but did not alter CO2 assimilation, gs, and transpiration rate in upper leaves and Ci in upper and middle leaves. Under Mg-deficiency, CO2 assimilation, gs, and transpiration rate decreased, but Ci increased with increasing leaf age. Under Mg-sufficiency, the four parameters displayed little change except for slightly decreased CO2 assimilation and transpiration rate and slightly increased Ci in lower leaves. Interactions between Mg and leaf positions were significant for the four parameters (Figure 3).
Figure 3.
Mg-deficiency effects on CO2 assimilation (a), stomatal conductance (gs, (b)), intercellular CO2 concentration (Ci, (c)), and transpiration rate (d) in C. sinensis leaves. Bars represent the mean ± standard error (n = 6). Data were analyzed by two-way ANOVA (three (leaf positions) × two (Mg levels)) followed by Duncan's multiple range. Different letters above the bars indicate a significant difference at p < 0.05. ** indicates a significant difference at p < 0.01. LP, leaf position.
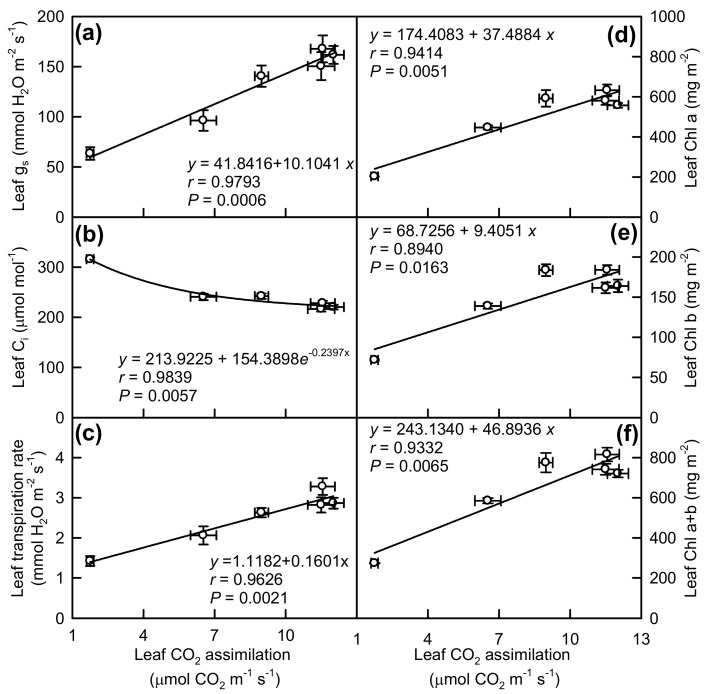
Leaf CO2 assimilation was positively related to gs, transpiration rate, Chl a, Chl b and Chl a + b concentration, respectively, but it was negatively related to Ci (Figure 4).
Figure 4.
CO2 assimilation in relation to gs (a), Ci (b), transpiration rate (c), Chl a (d), Chl b (e), and Chl a + b (f) in leaves. Point represents the mean ± standard error for the independent variable (n = 6) and the dependent variables (n = 4 or 6).
2.3. Leaf OJIP Transients and Related Parameters
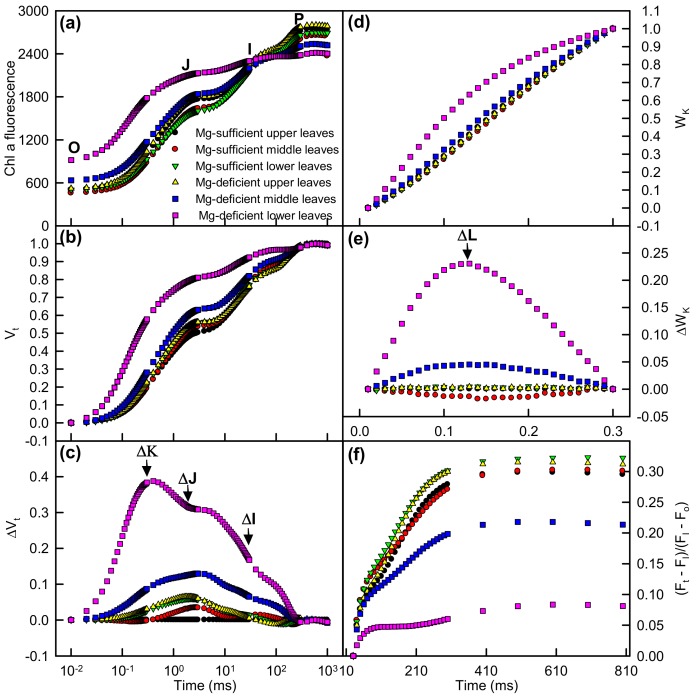
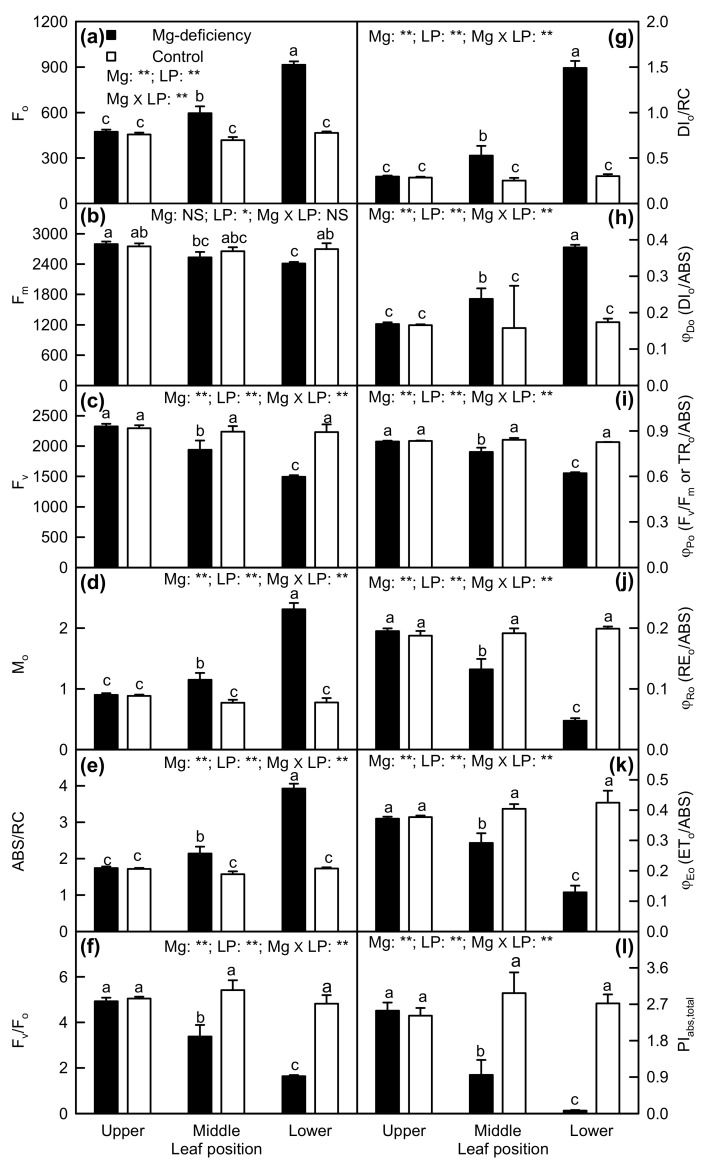
Compared with Mg-sufficient upper leaves, Mg-deficiency middle and upper leaves had an increased O-step and a decreased P-step, especially in Mg-deficiency lower leaves. Similarly, positive ΔL-, ΔK-, ΔJ-, and ΔI-bands and decreased maximum amplitude of IP phase [(Fm − FI)/(FI − Fo)] were observed only in Mg-deficiency middle and lower leaves, especially in the latter. Under Mg-sufficiency, leaf positions had little influence on OJIP transients (Figure 5).
Figure 5.
Mg-deficiency effects on the mean OJIP transients of four dark-adapted leaves (a) and the different expressions derived from the mean transients in dark-adapted leaves (b): between Fo and Fm: Vt = (Ft − Fo) / (Fm − Fo), and (c) the differences of samples to the control sample (Mg-sufficient upper leaves); (d) between Fo and F300µs: Wk = (Ft − Fo) / (F300µs − Fo) and (e) the differences of the samples to control sample, and (f) IP phase: (Ft − Fo) / (FI − Fo) − 1 = (Ft − FI) / (FI − Fo).
Minimum fluorescence (Fo), approximated initial slope (in ms-1) of the fluorescence transient V = f(t) (Mo), absorption flux per RC (ABS/RC), DIo/RC, and DIo/ABS were increased only in Mg-deficiency middle and lower leaves, especially in the latter, but maximum fluorescence (Fm), maximum variable fluorescence (Fv), Fv/Fm, maximum primary yield of photochemistry of PSII (Fv/Fo), quantum yield of electron transport from the reduced primary quinone electron acceptor of PSII (QA-) to the PSI end electron acceptors (REo/ABS), quantum yield for electron transport (ETo/ABS) and PIabs,total were decreased only in Mg-deficiency middle and lower leaves, especially in the latter. Under Mg-sufficiency, all these parameters were not significantly altered by leaf positions. Interactions between Mg and leaf positions were significant for all these parameters except for Fm (Figure 6).
Figure 6.
Mg-deficiency effects on Fo (a), Fm (b), Fv (c), Mo (d), ABS/RC (e), Fv/Fo (f), DIo/RC (g), DIo/ABS (h), Fv/Fm (i), REo/ABS (j), ETo/ABS (k) and PIabs,total (l) in C. sinensis leaves. Bars represent the mean ± standard error (n = 4). Data were analyzed by two-way ANOVA (three (leaf positions) × two (Mg levels)) followed by Duncan's multiple range. Different letters above the bars indicate a significant difference at p < 0.05. NS, *, and ** indicate nonsignificant, significant at 5% and 1% level, respectively. LP, leaf position.
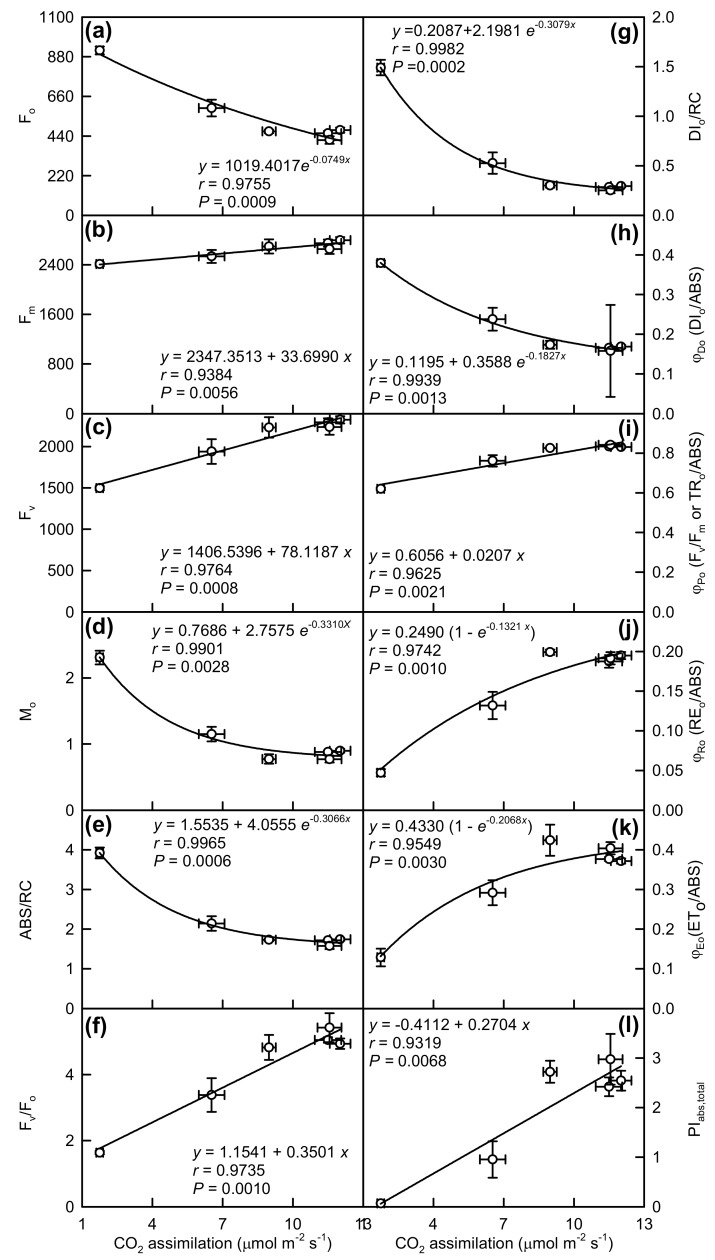
As shown in Figure 7, leaf CO2 assimilation was negatively related to Fo, Mo, ABS/RC, DIo/RC, and DIo/ABS, respectively, but it was positively related to Fm, Fv, Fv/Fo, Fv/Fm, REo/ABS, ETo/ABS, and PIabs,total, respectively.
Figure 7.
Leaf CO2 assimilation in relation to Fo (a), Fm (b), Fv (c), Mo (d), ABS/RC (e), Fv/Fo (f), DIo/RC (g), DIo/ABS (h), Fv/Fm (i), REo/ABS (j), ETo/ABS (k) and PIabs,total (l). Points represent means ± SE for the independent variable (n = 6) and the dependent variables (n = 4).
2.4. Element Concentrations in Leaf Blades and Veins
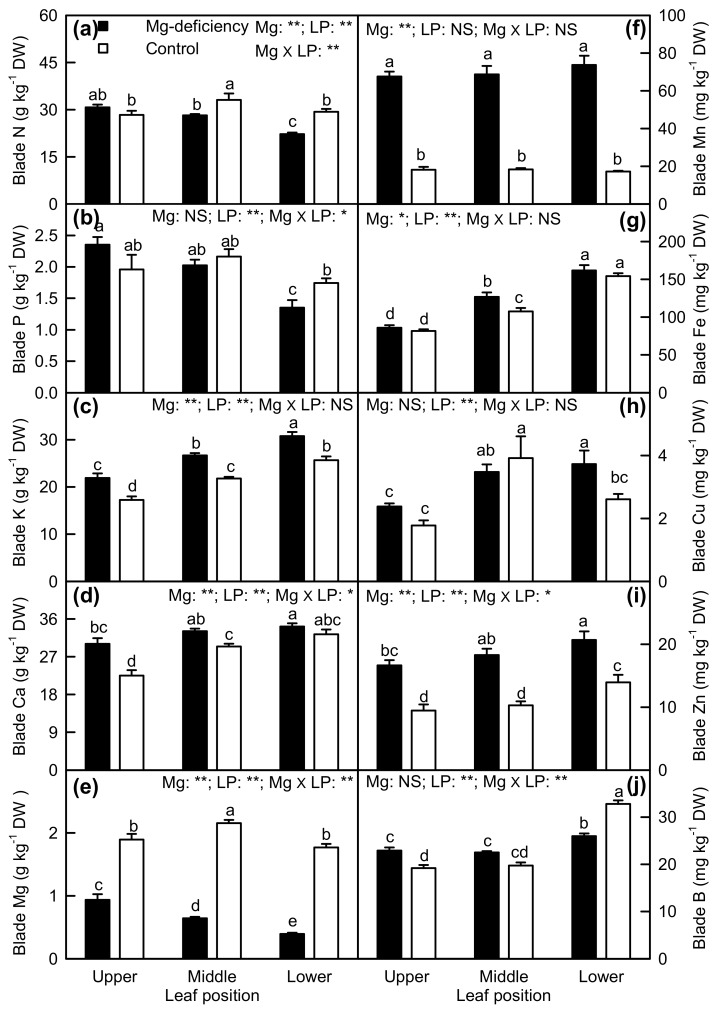
Mg-deficiency decreased or did not alter N, P, and Mg concentrations in the blades, but it increased or did not affect K, Ca, Mn, Fe, Cu, and Zn concentrations in the blades. B concentration was decreased in Mg-deficiency the blades of lower leaves, but it was increased in Mg-deficiency the blades of upper leaves. Under Mg-sufficiency, N, Mg, and Cu concentrations were significantly higher in the blades of middle leaves than those in the blades of upper and lower leaves; K, Ca, and Fe concentrations in leaf blades increased with increasing leaf age; Zn and B concentrations were higher in the blades of lower leaves than those in the blades of upper and middle leaves; P and Mn concentrations were similar among the blades of lower, middle, and upper leaves. Under Mg-deficiency, N, P, and Mg concentrations in the blades displayed a decreased trend with increasing leaf age; K, Ca, Fe, Cu, and Zn concentrations in the blades displayed an increasing trend with increasing leaf age; Mn concentration was similar among the blades of lower, middle and lower leaves; B concentration was higher in the blades of lower leave than that in the blades of upper and middle leaves. Interactions between Mg and leaf positions were significant only for the blade N, P, Ca, Mg, Zn, and B concentrations (Figure 8).
Figure 8.
Mg-deficiency effects on the concentrations of N (a), P (b), K (c), Ca (d), Mg (e), Mn (f), Fe (g), Cu (h), Zn (i) and B (j) in the blades of upper, middle, and lower leaves. Bars represent the mean ± standard error (n = 4–6). Data were analyzed by two-way ANOVA (three (leaf positions) × two (Mg levels)) followed by Duncan's multiple range. Different letters above the bars indicate a significant difference at p < 0.05. NS, *, and ** indicate nonsignificant, significant at 5% and 1% level, respectively. LP, leaf position.
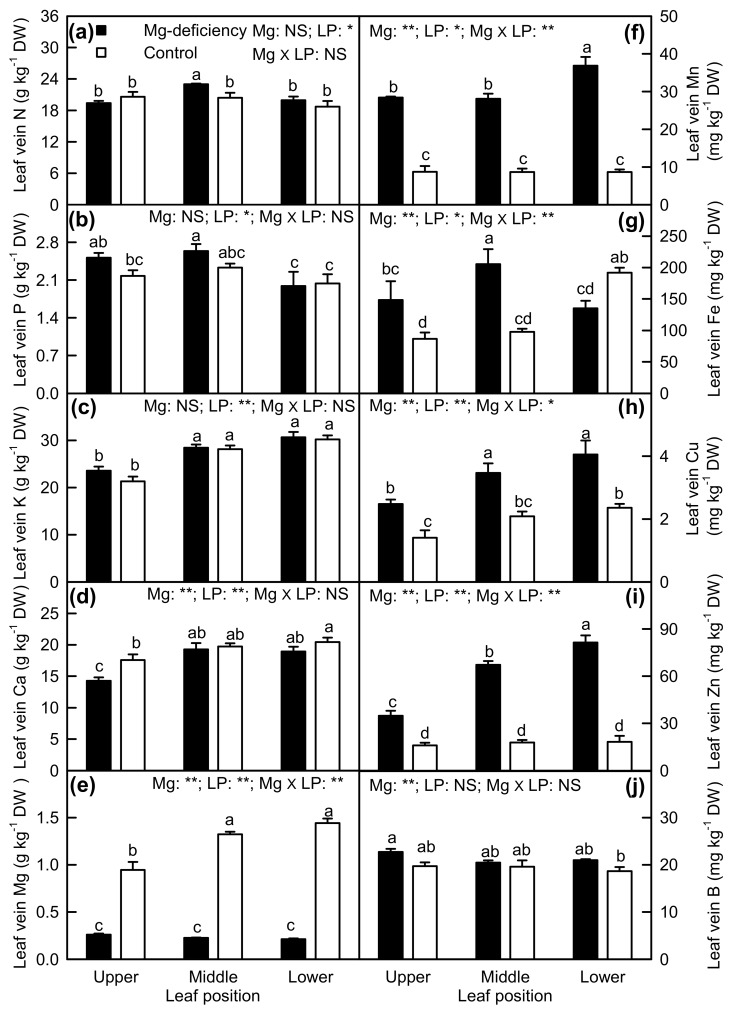
Magnesium-deficiency increased or had no significant influence on N, Mn, Cu, and Zn concentrations in veins, decreased or did not significantly alter Ca and Mg concentrations in veins, and did not significantly affect P, K, and B concentrations in veins. Mg-deficiency increased Fe concentration in veins of upper and middle leaves and decreased its concentration in veins of lower leaves. Under Mg-sufficiency, N, P, Mn, Zn, and B concentrations were similar among veins of upper, middle, and lower leaves; K and Mg concentrations were higher in veins of middle and lower leaves than those in veins of upper leaves; Fe concentration was higher in veins of lower leaves than those in veins of upper and middle leaves; Ca and Cu concentrations in veins increased with increasing leaf age. Under Mg-deficiency, N and Fe concentrations were higher in veins of middle leaves than those in veins of upper and lower leaves; P (Mn) concentration was higher in veins of upper and middle (lower) leaves than that in veins of lower (upper and middle) leaves; K, Ca, and Cu concentrations were higher in veins of middle and lower leaves than those in veins of upper leaves; Mg and B concentrations were similar among veins of upper, middle, and lower leaves; Zn concentration in veins increased with increasing leaf age. Interactions between Mg and leaf positions were significant only for Mg, Mn, Fe, Cu, and Zn concentrations (Figure 9).
Figure 9.
Mg-deficiency effects on the concentrations of N (a), P (b), K (c), Ca (d), Mg (e), Mn (f), Fe (g), Cu (h), Zn (i) and B (j) in the veins of upper, middle, and lower leaves. Bars represent the mean ± standard error (n = 4–6). Data were analyzed by two-way ANOVA (three (leaf positions) × two (Mg levels)) followed by Duncan's multiple range. Different letters above the bars indicate a significant difference at p < 0.05. NS, *, and ** indicate nonsignificant, significant at 5% and 1% level, respectively. LP, leaf position.
As shown in Table S1, a significant and positive relationship was observed between blade and vein for K, Mg, Mn, and Zn concentration, but not for N, P, Ca, Fe, Cu, or B concentration.
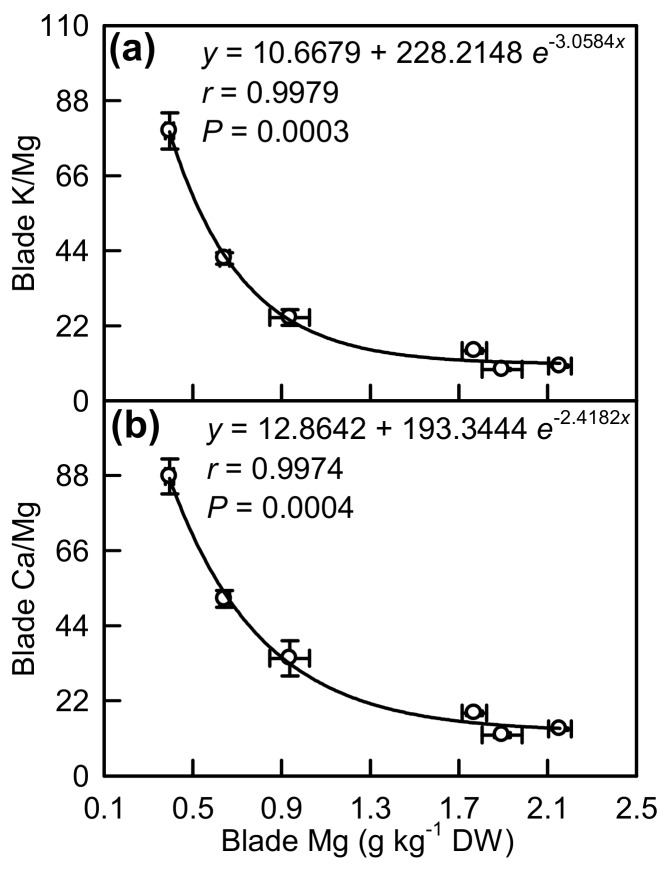
As shown in Figure 10, Mg concentration was negatively related to the K/Mg and Ca/Mg ratio in the blades, respectively.
Figure 10.
Mg concentration in relation to the K/Mg ratio (a) and Ca/Mg ratio (b) in the blades. Points represent the mean ± standard error for the independent variable (n = 6) and the dependent variables (n = 6).
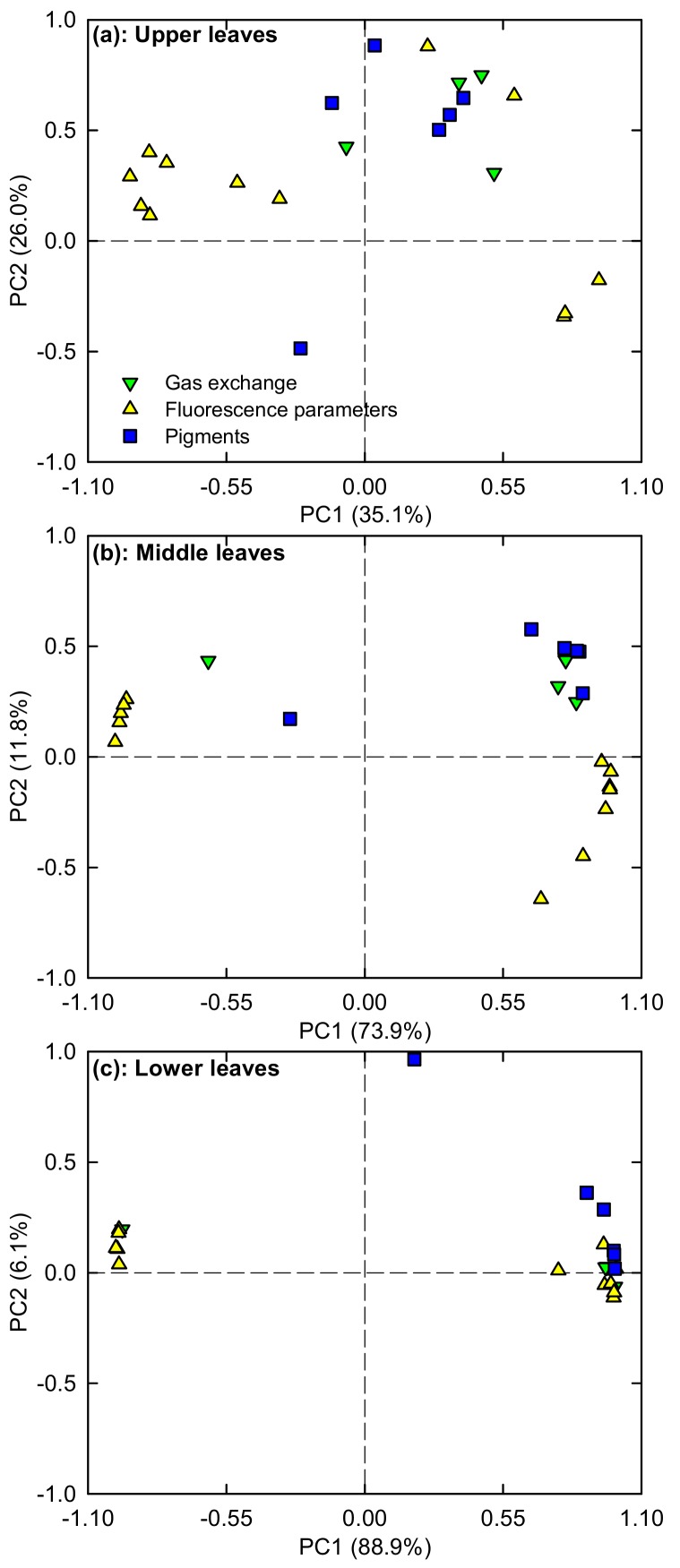
2.5. PCA Loading Plots
To determine the response patterns of photosynthesis and related physiological parameters (viz. gas exchange, pigments, and fluorescence parameters) in upper, middle, and lower leaves to Mg-deficiency, PCA was performed using these parameters (Figure 11 and Table S2). The first two components accounted for 61.1% (35.1% for PC1 and 26.0% for PC2), 85.7% (73.9% for PC1 and 11.8% for PC2), and 95.0% (88.9% for PC1 and 6.1% for PC2) of the total variation in upper, middle and lower leaves, respectively. Obviously, the contribution of PC1 and PC2 to the total variation increased with increasing leaf age.
Figure 11.
PCA loading plots of gas exchange, pigments and fluorescence parameters for upper (a), middle (b), and lower (c) leaves.
3. Discussion
3.1. Responses of Leaf Photosynthesis and Related Parameters to Mg-Deficiency Increased with Increasing Leaf Age, and Mg-Deficiency Increased Their Differences Among Leaves with Various Ages
We found that Mg-deficiency affected photosynthesis and related parameters more in lower leaves than those in middle leaves, but it had almost no influence on them in upper leaves (Figure 2, Figure 3, Figure 5 and Figure 6). PCA indicated that the contribution of PC1 and PC2 to the total variation increased with increasing leaf age (Figure 11), suggesting that Mg-deficiency-induced alterations of these physiological parameters as a whole increased with increasing leaf age. This was also supported by our observations that Mg-deficiency symptoms gradually extended from the basal old leaves toward the top young leaves, that interveinal chlorosis appeared in Mg-deficiency middle and lower leaves, especially in the latter, and that enlargement and corkiness of midribs and main lateral veins occurred in quite a few Mg-deficiency lower leaves and a few Mg-deficiency middle leaves, but not in Mg-deficiency upper leaves (Figure 1). Mg remobilization from the old leaves to the young leaves was enhanced under Mg-starved conditions [1]. Our results indicated that Mg concentration in the blades decreased with increasing leaf age under Mg-deficiency, but it kept relatively stable under Mg-sufficiency except that Mg concentration was slightly higher in the blades of middle leaves than that in the blades of upper and lower leaves (Figure 8e). This can explain why the Mg-deficiency-induced alterations of photosynthesis and related physiological parameters increased with increasing leaf age. In addition, the differences in photosynthesis and related physiological parameters among lower, middle, and lower leaves were increased by Mg-deficiency (Figure 2, Figure 3 and Figure 6). This might be related to our data that Mg-deficiency increased the differences in Mg concentration among the blades of the lower, middle, and upper leaves (Figure 8e).
3.2. Antagonistic Interactions Existed between Mg and Some Elements
The most striking Mg-deficiency effect on nutrient concentrations in the blades was the large increases in Mn and Zn concentrations (Figure 8f,i). This agrees with the studies performed in Brassica napus that Mg-deficiency stimulated the uptake of Mn and Zn by ~50% relative to their uptake expected from the growth rate [1], in maize that Mg-deficiency led to an increase in root and leaf Mn concentration [2], and in wheat [41] and potato [34] that Mn concentration was elevated in Mg-deficiency leaves. Our results showed that blade Mg concentration was negatively correlated with blade Mn and blade Zn concentration, respectively (Table S1), implying that an antagonistic interaction might exist between Mg and Mn (Zn). However, the antagonistic action of Mg on Mn uptake hardly existed in Medicago sativa [42]. It has been suggested that the competitive effects of Mg on Mn vary depending on plant species, concentrations of nutrients in the medium, and type of cultivation [2]. In potato leaves, Zn concentration did not increase in response to Mg deficiency [34]. The antagonistic effects of Mg on K and Ca uptake have been reported in Citrus [11,35] and onion [36]. As expected, we found that the concentrations of Ca and K were increased in Mg-deficiency the blades of upper, middle, and lower leaves with the only exception that Ca concentration did not significantly differ between Mg-deficiency and Mg-sufficiency blades of lower leaves (Figure 8c,d), as reported on C. sinensis leaves [33]. The competitive actions of Mg on K and Ca uptake were also supported by our findings that Mg concentration was negatively related to the K/Mg and Ca/Mg ratio in the blades, respectively (Figure 9).
3.3. Mg-Deficiency-Induced Alterations of Some Nutrient Concentrations Differed between Leaf Blades and Veins, and Mg-Deficiency-Induced Enlargement and Corkiness of Veins Were Not Caused by Mg-Deficiency-Induced B-Starvation
Although K, Mg, Mn, and Zn concentration in the blades had a significant and positive relationship with the corresponding element concentration in veins, respectively (Table S1), many differences existed in Mg-deficiency-induced alterations of nutrient concentrations between the two (Figure 8; Figure 9). For examples, Mg-deficiency decreased or did not significantly alter N and P concentrations in the blades, but it increased or had no significant influence on their concentrations in veins. Mg-deficiency increased K and Ca levels in the blades except for a similar Ca level between Mg-deficiency and Mg-sufficient blades of lower leaves, but it did not affect their concentration in veins with the exception that Ca concentration was decreased in veins of Mg-deficiency upper leaves. Mg-deficiency decreased or did not alter Fe and Cu concentrations in the blades, but it increased their concentrations in veins except that Fe concentration in veins of lower leaves was decreased by Mg-deficiency.
Similar to Citrus B-deficiency [40], enlargement and corkiness appeared in veins of Mg-deficiency lower and middle leaves (Figure 1). However, the symptoms were not caused by Mg-deficiency-induced B-starvation, since B concentration was similar between Mg-deficiency and Mg-sufficiency veins and among veins of the lower, middle, and upper leaves (Figure 9j).
3.4. Impairment of Photosynthetic Electron Transport Chain Might Contribute to Mg-Deficiency-Induced Decline in Leaf CO2 Assimilation
The observed increased Ci in Mg-deficiency middle and lower leaves (Figure 3c) and the negative relationship between leaf CO2 assimilation and Ci (Figure 4b) indicated that Mg-deficiency-induced decline in leaf CO2 assimilation (Figure 3a) was mainly associated with non-stomatal factors, as found for longan [13], Citrus [3,12,43,44], and Norway spruce [45]. Peaslee and Moss suggested that Mg-deficiency inhibited maize leaf photosynthesis through Chl deterioration because chlorosis preceded the decline in photosynthesis [46]. Despite the negative relationship between Chl a + b concentration and CO2 assimilation in leaves (Figure 4f), the observed lower CO2 assimilation in Mg-deficiency middle and lower leaves (Figure 3a) might not be explained by the decreased Chl a + b level alone, since there was a far less decrease in leaf Chl a + b level than in CO2 assimilation (Figure 2c and Figure 3a). This is also supported by our findings showing that both DIo/RC and DIo/ABS were increased in Mg-deficiency middle and lower leaves (Figure 6g,h).
Excessive accumulation of soluble sugars (especially hexose) and starch can indirectly repress the expression of the genes encoding photosynthetic enzymes [47], and directly damage chloroplast function [48], respectively, thus inhibiting photosynthesis. The observed enlargement and corkiness of midribs and main lateral veins in Mg-deficiency middle and lower leaves indicated that Mg-deficiency impaired the structure of phloem tissue in both midribs and main lateral veins, which might block the transport of photoassimilates, thus leading the accumulation of nonstructural carbohydrates [12,33]. However, Mg-deficiency-induced inhibition of photosynthesis in middle and lower leaves could not explained alone by feedback inhibition due to the accumulation of nonstructural carbohydrates, because the concentrations of glucose, fructose, sucrose, total soluble sugars (glucose + fructose + sucrose), starch and total nonstructural carbohydrates (total soluble sugars + starch) did not significantly differ between Mg-deficiency upper and lower leaves in C. sinensis seedlings [3].
The observed positive ΔL-band in Mg-deficiency middle and lower leaves (Figure 5e) implied that the energetic connectivity (grouping) between PSII units was decreased in these leaves [21,30], especially in Mg-deficiency lower leaves. The lower grouping suggested that the PSII units of these leaves were less stable and became more fragile [31]. The positive ΔL-band has been observed in N-, P-, K-, Ca-, Mg-, S-, and Fe-deficiency maize and tomato [20], N-limited cowpea [30] and radish [49], P-starved tea [26], K-, Ca-, and Fe-deficiency maize [25], Mg- [12,43] and B-deficiency Citrus [19], and Fe-deficiency rapeseed [21].
The positive ΔK-band which occurred in Mg-deficiency middle and lower leaves (Figure 5c) indicated that OEC (especially of the Mn complex on PSII donor side) was inactivated and antennae complexes were less-connected possibly due to improper organism of membranes, thus decreasing energy transfer and absorption efficiency in these leaves, especially in Mg-deficiency lower leaves [21,50,51]. Positive ΔK-band has also been observed in all nutrient-deficiency maize and tomato [20], N-deficiency radish [49], K-, Ca-, and Fe-deficiency maize [25], Fe-deficiency rapeseed [21], B- [19] and Mg-starved Citrus [12,43], and P-deficiency tea [26].
We found that ABS/RC increased in Mg-deficiency middle and lower leaves, with a greater increase in the latter (Figure 6c), as found in N-deficiency radish [49], K-, Ca-, and Fe-deficiency maize and bean [25], N-, K-, Ca-, Mg-, and S-deficiency maize, N-, K-, Ca-, Mg-, and Fe-deficiency potato [20], and B-starved Citrus [19]. However, ABS/RC was decreased in Fe-deficiency rapeseed [21], and unaltered in P- and S-deficiency tomato, and P- and Fe-deficiency maize [20]. The observed higher ABS/RC implied that either a part of RCs was inactivated or the apparent antenna size was elevated [20,25]. The decrease in active RCs was also supported by the increased closure rate of RCs (Mo) (Figure 6d) [49]. The inactivation of RCs is suggested to be a downregulation (adaptive) mechanism that protects the nutrient-starved leaves against photo-oxidative damage through dissipating the excess absorbed light energy [20,25]. Indeed, energy dissipation was upregulated in Mg-deficiency middle and lower leaves, as indicated by the increased DIo/RC and DIo/ABS (Figure 6g,h).
ΔJ-band, ΔI-band, and IP phase (a measure of the amount of reduced end acceptors at PSI acceptor side, the last and slowest rate-limiting step of the photosynthetic electron transport chain) are associated with the reduction of QA, the reduction of plastoquinone pool, and the reduction of the electron transport acceptors in and around PSI [20,26,52]. The positive ΔJ- and ΔI-bands, and the decreased maximum amplitude of IP phase in Mg-deficiency middle and lower leaves (Figure 5c,f) suggested that Mg-deficiency increased the reduction of PSII acceptor side and the oxidation of PSI acceptor side. In other words, PSII was a more sensitive Mg-deficiency-inhibited site than PSI; Mg-deficiency affected the donor side of PSII less than the acceptor side of PSII [12,26,30,53]. This agrees with the in vitro study showing that the inactivation of PSII acceptor side was the main site for the impairment of electron transport [54]. Mg-deficiency-induced damage of PSII acceptor side was further supported by decreased Fv and increased Fo, the characteristic of photoinhibitory impairment at PSII acceptor side [55]. Increased VJ and VI and decreased maximum amplitude of IP phase have been obtained in P-starved tea [26], and B- [19] and Mg-deficiency Citrus [12,43].In a study, Kalaji et al. observed positive O-J and J-I normalized bands, but negative I-P normalized bands in moderate and strong Fe-deficiency rapeseed leaves, they suggested that this might be a compensatory mechanism [21]. Positive ΔJ- and ΔI-bands were observed in all nutrient-deficiency maize and tomato [20], Ca- and Fe-deficiency maize [25], and N-deficiency radish [49]. Unfortunately, the IP phase (I-P normalized band) was not provided in these studies.
Magnesium-deficiency decreased Fv/Fm (a good indicator of photoinhibitory effects on PSII), ETo/ABS and probability that a trapped exciton moves an electron into the electron transport chain beyond QA− (ETo/TRo = 1 − VJ), increased DIo/RC, and altered greatly OJIP transients in middle and lower leaves (Figure 5 and Figure 6), together demonstrating that photoinhibition occurred in Mg-deficiency middle and lower leaves [21,56,57]. Nutrient deficiency-induced photoinhibitory damage to PSII has also been obtained on nutrient-deficiency Citrus [12,19,43], tea [26], maize, tomato [20,25], rapeseed [21], and radish [49]. The lower Fv/Fm in Mg-deficiency middle and lower leaves was caused by both elevated Fo and reduced Fm, especially by the former (Figure 6a,b). The elevated Fo might be due to accumulation of QA−, as indicated by increased Mo (Figure 6d) or the impairment of OEC, as indicated by the positive ΔK-band (Figure 5e).
We observed that REo/ABS (the quantum yield with which electrons reduce the PSI end-electron acceptors) and PIabs,total (a measure for the performance up to reduction of PSI end-electron acceptors) were decreased in Mg-deficiency middle and lower leaves, especially in the latter (Figure 6j,l), indicating that the reduction of PSI end-electron acceptors was impaired in these leaves. Similar results have been obtained in N-deficiency radish [49], moderate and strong Fe-deficiency rapeseed [21], B- [19], and Mg-deficiency Citrus [12,43], Ca- and Fe-deficiency maize [25], and P-deficiency tea [26]. However, Aleksandrov et al. observed that K-, Ca-, and Fe-deficiency bean leaves had decreased REo/ABS, but increased PIabs,total [25]. Kalaji et al. reported that REo/ABS was decreased in Fe-, K-, Mg-, and S-deficiency maize (Fe-deficiency tomato), and PIabs,total was decreased in Fe-, K-, Mg-, N-, S-, and Ca-deficiency maize (Fe-, N- and S-deficiency tomato), but REo/ABS and PIabs,total were not significantly affected in the other nutrient-deficiency maize and tomato [20].
The decreased Fv/Fo in Mg-deficiency middle and lower leaves (Figure 6f) indicated that Mg-deficiency might damage the structure of thylakoids, thus inhibiting the photosynthetic electron transport [58]. This is also supported by the previous reports that Mg-deficiency damage chloroplast ultrastructure in Citrus leaves [59,60]. Decreased Fv/Fo has been observed on Fe-, K-, and Ca-deficiency maize, bean [25], N-deficiency radish [49], B-stressed Citrus [19], P-starved tea [26], Mg-deficiency Citrus [12,43], Mn-toxicity Citrus [61], low pH-stressed Citrus [62,63], and Al-toxicity Citrus [31,53,64]. The decreased Fv/Fo in Mg-deficiency middle and lower leaves was due to both decreased Fv and increased Fo (Figure 6a,c,f).
Based on these results and above discussion (Figure 6 and Figure 7), we concluded that the impairment of the whole photosynthetic electron transport chain from the donor side of PSII to the reduction of PSI end-electron acceptors (viz. structural damage to thylakoids, ungrouping of PSII units, inactivation of OEC and RCs, increased reduction of QA and plastoquinone pool at PSII acceptor side and oxidation of PSI end-electron acceptors) might be responsible for the decline in CO2 assimilation in Mg-deficiency middle and lower leaves.
4. Materials and Methods
4.1. Plant Culture and Mg Treatments
Plant culture and Mg treatments were made according to Cai et al. [8]. Briefly, uniform 5-week-old ‘Xuegan’ (C. sinensis) seedlings with single stem were transplanted to 6 L pots (two plants per pot) containing sands washed thoroughly with tap water, then cultivated in a greenhouse with a natural photoperiod at the Fujian Agriculture and Forestry University. Ten weeks after transporting, each pot was irrigated with nutrient solution (i.e., macronutrients (in mM): Ca(NO3)2, 5; K2SO4, 2; KH2PO4, 1; KNO3, 1; and micronutrients (in μM): Fe-EDTA, 20; H3BO3, 10; ZnSO4, 2; MnCl2, 2; CuSO4, 0.5, and (NH4)6Mo7O24, 0.065) at a concentration of 0 (Mg-deficiency) or 2 (control) mM Mg(NO3)2 every two days until a portion of solution leaked out from a hole in the bottom of the pot (about 500 mL). N in the nutrient solution was kept at a constant concentration by supplying equivalent moles of NH4NO3 instead of Mg(NO3)2. Sixteen weeks after treatment, upper three-quarter height, ~5-week-old), middle (two-quarter height, ~8-week-old), and lower (three-quarter height, ~11-week-old) leaves were used for all the measurements. Upper, middle, and lower leaves were fully expanded mature leaves [3].
4.2. Leaf Pigments and Gas Exchange
Leaf Chl a, Chl b, and Car) were determined according to Lichtenthaler after being extracted with 80 (v/v) acetone [65].
Leaf gas exchange was made with a CIRAS-2 portable photosynthesis system (PP Systems, Herts, UK) at a leaf temperature of 30 ± 0.2 °C, a relative humidity of 64.5 ± 0.6%, a controlled CO2 concentration of ~380 μmol mol−1, and a controlled light intensity of ~1000 μmol m−2 s−1 between 9:30 and 11:00 a.m. on a sunny day [66]. There were six replicates per treatment.
4.3. Leaf OJIP Transients and Related Parameters
Leaf OJIP transients were measured by a Handy PEA (Hansatech Instruments Limited, Norfolk, UK) after seedlings were dark-adapted for 3 h at room temperature. All fluorescence parameters were calculated according to Jiang et al. [31], Chen and Cheng [67], and Bank [68]. There were four replicates per treatment.
4.4. Assays of Elements
Lower, middle, and lower leaves were collected. Midribs and main lateral veins were carefully cut from the leaves with sharp medical scissors. A small number of blades adhered to the veins were scraped with a single knife. Then, blades (petioles, midribs, and veins removed) were collected. After being dried to a constant weight at 70 °C, all samples were ground, sieved (40-mesh), and properly stored for analysis. P was assayed colorimetrically as the blue molybdate–phosphate complexes according to Li et al. [11]. K was determined with an FP640 flame photometry (Shanghai Precision Scientific Instrument Co., Ltd, Shanghai, China). Ca and Mg were measured with a PinAAcle 900F atomic absorption spectrometer (PerkinElmer Singapore Pte Ltd, Singapore). Fe, Mn, Cu, Zn, and B were assayed with a NexION 300X inductively coupled plasma mass spectrometer (ICP-MS, PerkinElmer, Shelton, CT, USA). N was determined using an elementary Vario Max Cube Analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). There were three to six replicates per treatment.
4.5. Statistical Analysis
There were 20 pots (40 plants) per treatment in a completely randomized design. Results were the means ± SE (n = 4–6) (one plant from different pots per replicate with the exception of element analysis (four plants from two pots per replicate)) with the exception of the mean OJIP transient (only means). Data were analyzed by two-way ANOVA (three (leaf positions) × two (Mg levels)) followed by Duncan’s multiple range.
Principal component analysis (PCA) was carried out with an SPSSstatistical software (version 17.0, IBM, NY, USA) [66,69].
5. Conclusions
Our findings clearly demonstrated that Mg-deficiency-induced interveinal chlorosis, vein enlargement, and corkiness, and alterations of photosynthesis and related parameters were observed in middle and lower leaves, especially in the latter, but not in upper leaves. This might be related to the increased remobilization of Mg from the old leaves to the young leaves under Mg-starvation, since Mg concentration in the blades decreased with increasing leaf age. Antagonistic interactions existed between Mg and some elements. Although blade K, Mg, Mn, and Zn concentration displayed a significant and positive relationship with the corresponding vein nutrient concentration, respectively, great differences existed in Mg-deficiency-induced alterations of nutrient concentrations between leaf blades and veins. Mg-deficiency-induced enlargement and corkiness of veins were not caused by Mg-deficiency-induced B-starvation. Mg-deficiency might impair the whole photosynthetic electron transport, including structural damage to thylakoids, ungrouping of PSII units, inactivation of OEC and RCs, increased reduction of QA and plastoquinone pool at PSII acceptor side, and oxidation of PSI end-electron acceptors, thus decreasing energy transfer and absorption efficiency and the transfer of electrons to the dark reactions, and hence, the rate of CO2 assimilation in Mg-deficiency middle and lower leaves.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/8/10/389/s1, Table S1: Pearson correlation coefficient matrix for the concentrations of elements in leaves and leaf veins, Table S2: PCA for leaf gas exchange and related physiological parameters of upper, middle and lower leaves.
Author Contributions
Conceptualization, X.Y., C.-L.D., L.-T.Y., and L.-S.C.; methodology, X.Y., and X.-F.C.; validation, X.Y., and X.-F.C.; formal analysis, X.Y., C.-L.D., J.-X.G., and L.-S.C.; investigation, X.Y., and X.-F.C.; resources, X.Y., and L.-S.C.; data curation, X.Y., and N.-W.L.; writing—original draft preparation, X.Y.; writing—review and editing, L.-S.C.; supervision, L.-S.C.; project administration, L.-S.C.; funding acquisition, L.-S.C., and X.Y.
Funding
This study was financially supported by the Provincial Natural Science Foundation of Fujian, China (No. 2016J01115), the National Natural Science Foundation of China (No. 31572081), the earmarked fund for China Agriculture Research System (No. CARS27), and the Special Fund for Scientific and Technological Innovation of Fujian Agriculture and Forestry University (No.CXZX2017358), and the Opening Foundation of Key Lab of Soil Ecosystem Health and Regulation (Fujian Agriculture and Forestry University), Fujian Province University. The APC was funded by the National Natural Science Foundation of China (No. 31572081).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Billard V., Maillard A., Coquet L., Jouenne T., Cruz F., Garcia-Mina J.M., Yvin J.-C., Ourry A., Etienne P. Mg deficiency affects leaf Mg remobilization and the proteome in Brassica napus. Plant Physiol. Biochem. 2016;107:337–343. doi: 10.1016/j.plaphy.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Jezek M., Geilfus C.M., Bayer A., Mühling K.H. Photosynthesis capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2015;5:781. doi: 10.3389/fpls.2014.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C.-P., Qi Y.-P., Zhang J., Yang L.-T., Wang D.-H., Ye X., Lai N.-W., Tan L.-L., Li D., Chen L.-S. Magnesium-deficiency-induced alterations of gas exchange, major metabolites and key enzymes differ among roots, lower and upper leaves of Citrus sinensis seedlings. Tree Physiol. 2017;37:1564–1581. doi: 10.1093/treephys/tpx067. [DOI] [PubMed] [Google Scholar]
- 4.Peng H.-Y., Qi Y.-P., Lee J., Yang L.-T., Guo P., Jiang H.-X., Chen L.-S. Proteomic analysis of Citrus sinensis roots and leaves in response to long-term magnesium-deficiency. BMC Genom. 2015;16:253. doi: 10.1186/s12864-015-1462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma C.-L., Qi Y.-P., Liang W.-W., Yang L.-T., Lu Y.-B., Guo P., Ye X., Chen L.-S. MicroRNA regulatory mechanisms on Citrus sinensis leaves to magnesium-deficiency. Front. Plant Sci. 2016;7:201. doi: 10.3389/fpls.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cakmak I., Yazici A.M. Magnesium: A forgotten element in crop production. Better Crops. 2010;94:23–25. [Google Scholar]
- 7.Verbruggen N., Hermans C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil. 2013;368:87–99. doi: 10.1007/s11104-013-1589-0. [DOI] [Google Scholar]
- 8.Cai Y.-T., Zhang H., Qi Y.-P., Ye X., Huang Z.-R., Guo J.-X., Chen L.-S., Yang L.-T. Responses of reactive oxygen species and methylglyoxal metabolisms to magnesium-deficiency differ greatly among the roots, upper and lower leaves of Citrus sinensis. BMC Plant Biol. 2019;19:76. doi: 10.1186/s12870-019-1683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W., Hussain H., Liang Z., Yang D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016;4:83–91. doi: 10.1016/j.cj.2015.11.003. [DOI] [Google Scholar]
- 10.Ling L.-L., Peng L.-Z., Cao L., Jiang C.-L., Chun C.-P., Zhang G.-Y., Wang Z.-X. Effect of magnesium deficiency on photosynthesis characteristic of Beibei 447 Jinchen orange. J. Fruit Sci. 2009;26:275–280. [Google Scholar]
- 11.Li Y., Han M.-Q., Lin F., Ten Y., Lin J., Zhu D.-H., Guo P., Weng Y.-B., Chen L.-S. Soil chemical properties, ‘Guanximiyou’ pummelo leaf mineral nutrient status and fruit quality in the southern region of Fujian province, China. J. Soil Sci. Plant Nutr. 2015;15:615–628. doi: 10.4067/S0718-95162015005000029. [DOI] [Google Scholar]
- 12.Yang G.-H., Yang L.-T., Jiang H.-X., Wang P., Chen L.-S. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees Struct. Funct. 2012;26:1237–1250. doi: 10.1007/s00468-012-0699-2. [DOI] [Google Scholar]
- 13.Li Y., Liu X.-H., Zhuang W.-M. The effect of magnesium deficiency on photosynthesis of longan (Dimocarpus longan Lour.) seedlings. Acta Hortic. Sin. 2001;28:101–106. [Google Scholar]
- 14.Hermans C., Verbruggen N. Physiological characterization of magnesium deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005;56:2153–2161. doi: 10.1093/jxb/eri215. [DOI] [PubMed] [Google Scholar]
- 15.da Silva D.M., Brandão I.R., Alves J.D., de Santos M.O., de Souza K.R.D., de Silveira H.R.O. Physiological and biochemical impacts of magnesium-deficiency in two cultivars of coffee. Plant Soil. 2014;382:133–150. doi: 10.1007/s11104-014-2150-5. [DOI] [Google Scholar]
- 16.Ceylan Y., Kutman U.B., Mengutay M., Cakmak I. Magnesium applications to growth medium and foliage affect the starch distribution, increase the grain size and improve the seed germination in wheat. Plant Soil. 2016;406:145–1561. doi: 10.1007/s11104-016-2871-8. [DOI] [Google Scholar]
- 17.Fischer E.S., Lohaus G., Heineke D., Heldt H.W. Magnesium deficiency results in accumulation of carbohydrates and amino acids in source and sink leaves of spinach. Physiol. Plant. 1998;102:16–20. doi: 10.1034/j.1399-3054.1998.1020103.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi N.I., Saito T., Iwata N., Ohmae Y., Iwata R., Tanoi K., Nakanishi T.M. Leaf senescence in rice due to magnesium deficiency mediated defect in transpiration rate before sugar accumulation and chlorosis. Physiol. Plant. 2013;148:490–501. doi: 10.1111/j.1399-3054.2012.12003.x. [DOI] [PubMed] [Google Scholar]
- 19.Han S., Tang N., Jiang H.-X., Yang L.-T., Li Y., Chen L.-S. CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of Citrus leaves in response to boron stress. Plant Sci. 2009;176:143–153. doi: 10.1016/j.plantsci.2008.10.004. [DOI] [Google Scholar]
- 20.Kalaji H.M., Oukarroum A., Alexandrov V., Kouzmanova M., Brestic M., Zivcak M., Samborska I.A., Cetner M.D., Allakhverdiev S.I., Goltsev V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014;81:16–25. doi: 10.1016/j.plaphy.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Kalaji H.M., Bąba W., Gediga K., Goltsev V., Samborska I.A., Cetner M.D., Dimitrovam S., Piszcz U., Bielecki K., Karmowska K., et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018;136:329–343. doi: 10.1007/s11120-017-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasarao C., Shanker A.K., Kundu S., Reddy S. Chlorophyll fluorescence induction kinetics and yield responses in rainfed crops with variable potassium nutrition in K deficient semi-arid alfisols. J. Photochem. Photobiol. B. 2016;160:86–95. doi: 10.1016/j.jphotobiol.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Carstensen A., Herdean A., Schmidt S.B., Sharma A., Spetea C., Pribil M., Husted S. The mmpacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 2018;177:271–284. doi: 10.1104/pp.17.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carstensen A., Szameitat A.E., Frydenvang J., Husted S. Chlorophyll a fluorescence analysis can detect phosphorus deficiency under field conditions and is an effective tool to prevent grain yield reductions in spring barley (Hordeum vulgare L.) Plant Soil. 2019;434:79–91. doi: 10.1007/s11104-018-3783-6. [DOI] [Google Scholar]
- 25.Aleksandrov V., Krasteva V., Paunov M., Chepisheva M., Kousmanova M., Kalaji H.M., Goltsev V. Deficiency of some nutrient elements in bean and maize plants analyzed by luminescent method. Bulg. J. Agric. Sci. 2014;20:24–30. [Google Scholar]
- 26.Lin Z.-H., Chen L.-S., Chen R.-B., Zhang F.-Z., Jiang H.-X., Tang N. CO2 assimilation, ribulose-1, 5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol. 2009;9:43. doi: 10.1186/1471-2229-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt S.B., Pedas P., Laursen K.H., Schjoerring J.K., Husted S. Latent manganese deficiency in barley can be diagnosed and remediated on the basis of chlorophyll a fluorescence measurements. Plant Soil. 2013;372:417–429. doi: 10.1007/s11104-013-1702-4. [DOI] [Google Scholar]
- 28.Kalaji H.M., Schansker G., Ladle R.J., Goltsev V., Bosa K., Allakhverdiev S.I., Brestic M., Bussotti F., Calatayud A., Dąbrowski P., et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014;122:121–158. doi: 10.1007/s11120-014-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalaji H.M., Schansker G., Brestic M., Bussotti F., Calatayud A., Ferroni L., Goltsev V., Guidi L., Jajoo A., Li P., et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017;132:13–66. doi: 10.1007/s11120-016-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strasser R.J., Tsimilli-Micheal M., Srivastava A. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou G.C., editor. Chlorophyll a Fluorescence: A Signature of Photosynthesis. Volume 19. Springer; Berlin, Germany: 2004. pp. 321–362. [Google Scholar]
- 31.Jiang H.-X., Chen L.-S., Zheng J.-G., Han S., Tang N., Smith B.R. Aluminum-induced effects on photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008;28:1863–1871. doi: 10.1093/treephys/28.12.1863. [DOI] [PubMed] [Google Scholar]
- 32.Živčák M., Olšovská K., Slamka P., Galambošová J., Rataj V., Shao H.B., Brestič M. Application of chlorophyll fluorescence performance indices to assess the wheat photosynthetic functions influenced by nitrogen deficiency. Plant Soil Environ. 2015;60:210–215. doi: 10.17221/73/2014-PSE. [DOI] [Google Scholar]
- 33.Huang J.-H., Xu J., Ye X., Luo T.-Y., Ren L.-H., Fan G.-C., Qi Y.-P., Li Q., Ferrarezi R.S., Chen L.-S. Magnesium deficiency affects secondary lignification of the vascular system in Citrus sinensis seedlings. Trees Struct. Funct. 2019;33:171–182. doi: 10.1007/s00468-018-1766-0. [DOI] [Google Scholar]
- 34.Cao W.X., Tibbitts T.W. Growth, carbon dioxide exchange and mineral accumulation in potatoes grown at different magnesium concentrations. J. Plant Nutr. 1992;15:1359–1371. doi: 10.1080/01904169209364403. [DOI] [PubMed] [Google Scholar]
- 35.Moss G.I., Higgins M.L. Magnesium influences on the fruit quality of sweet orange (Citrus sinensis L. Osbeck) Plant Soil. 1974;41:103–112. doi: 10.1007/BF00017948. [DOI] [Google Scholar]
- 36.Kleiber T., Golcz A., Krzesinski W. Effect of magnesium nutrition on onion (Allium cepa L.). Part, I. Yielding and nutrient status. Ecol. Chem. Eng. 2012;19:97–105. doi: 10.2478/v10216-011-0010-2. [DOI] [Google Scholar]
- 37.Alim K., Frey E. Quantification of leaf vein patterning. Biophys. J. 2009;96:631a. doi: 10.1016/j.bpj.2008.12.3339. [DOI] [Google Scholar]
- 38.Zhao H., Wu L., Chai T., Zhang Y., Tan J., Ma S. The effects of copper, manganese and zinc on plant growth and elemental accumulation in the manganese-hyperaccumulator Phytolacca americana. J. Plant Physiol. 2012;169:1243–1252. doi: 10.1016/j.jplph.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Vogel-Mikuš K., Simčič J., Pelicon P., Budnar M., Kump P., Nečemer M., Mesjasz-Przybyłowicz J., Przybyłowicz W.J., Regvar M. Comparison of essential and non-essential element distribution in leaves of the Cd/Zn hyperaccumulator Thlaspi praecox as revealed by micro-PIXE. Plant Cell Environ. 2008;31:1484–1496. doi: 10.1111/j.1365-3040.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 40.Han S., Chen L.-S., Jiang H.-X., Smith B.R., Yang L.-T., Xie C.-Y. Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of Citrus seedlings. J. Plant Physiol. 2008;165:1331–1341. doi: 10.1016/j.jplph.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Chatterjee C., Nautiyal N., Agarwala S.C. Influence of changes in manganese and magnesium supply on some aspects of wheat physiology. Soil Sci. Plant Nutr. 1994;40:191–197. doi: 10.1080/00380768.1994.10413293. [DOI] [Google Scholar]
- 42.Löhnis M.P. Effect of magnesium and calcium supply on the uptake of manganese by various crop plants. Plant Soil. 1960;12:339–376. doi: 10.1007/BF02232990. [DOI] [Google Scholar]
- 43.Tang N., Li Y., Chen L.-S. Magnesium deficiency-induced impairment of photosynthesis in leaves of fruiting Citrus reticulata trees accompanied by up-regulation of antioxidant metabolism to avoid photooxidative damage. J. Plant Nutr. Soil Sci. 2012;175:784–793. doi: 10.1002/jpln.201100329. [DOI] [Google Scholar]
- 44.Jin X.-L., Ma C.-L., Yang L.-T., Chen L.-S. Alterations of physiology and gene expression due to long-term magnesium-deficiency differ between leaves and roots of Citrus reticulata. J. Plant Physiol. 2016;198:103–115. doi: 10.1016/j.jplph.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Mehne-Jakobs B. Magnesium deficiency treatment causes reductions in photosynthesis of well-nourished Norway spruce. Trees Struct. Funct. 1996;10:293–300. doi: 10.1007/BF02340775. [DOI] [Google Scholar]
- 46.Peaslee D.-E., Moss D.N. Photosynthesis in K- and Mg-deficient maize (Zea mays L.) leaves. Soil Sci. Soc. Am. J. 1966;30:220–223. doi: 10.2136/sssaj1966.03615995003000020023x. [DOI] [Google Scholar]
- 47.Sheen J. Feedback control of gene expression. Photosynth. Res. 1994;39:427–438. doi: 10.1007/BF00014596. [DOI] [PubMed] [Google Scholar]
- 48.Cave G., Tolley L.C., Strain B.R. Effect of carbon dioxide enrichment on chlorophyll content, starch content and starch grain structure in Trifolium subteraneum leaves. Physiol. Plant. 1981;51:171–174. doi: 10.1111/j.1399-3054.1981.tb02694.x. [DOI] [Google Scholar]
- 49.Cetner M.D., Kalaji H.M., Goltsev V., Aleksandrov V., Kowalczyk K., Borucki W., Jajoo A. Effects of nitrogen-deficiency on efficiency of light-harvesting apparatus in radish. Plant Physiol. Biochem. 2017;119:81–92. doi: 10.1016/j.plaphy.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava A., Guisse B., Greppin H., Strasser R.J. Regulation of antenna structure and electron transport in photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim. Biophys. Acta. 1997;1320:95–106. doi: 10.1016/S0005-2728(97)00017-0. [DOI] [Google Scholar]
- 51.Hakala M., Tuominen I., Kerӓnen M., Tyystjӓrvi T., Tyystjӓrvi E. Eviddence for the role of the oxygen-evolving manganese complex in photoinhibition of photosystem II. Biochim. Biophys. Acta. 2005;1706:68–80. doi: 10.1016/j.bbabio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Schansker G., Tüth S.Z., Strasser R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta. 2005;1706:250–261. doi: 10.1016/j.bbabio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Liao X.-Y., Yang L.-T., Lu Y.-B., Ye X., Chen L.-S. Roles of rootstocks and scions in aluminum-tolerance of Citrus. Acta Physiol. Plant. 2015;37:1743. doi: 10.1007/s11738-014-1743-1. [DOI] [Google Scholar]
- 54.Aro E.-M., McCaffery S., Anderson J.M. Photoinhibition and D1 protein degradation in peas acclimated to different growth irradiances. Plant Physiol. 1993;103:835–843. doi: 10.1104/pp.103.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Šetlík I., Allakhverdiev S.I., Nedbal L., Šetlíková E., Klimov V.V. Three types of photosystem II photoinactivation. Photosynth. Res. 1990;23:39–48. doi: 10.1007/BF00030061. [DOI] [PubMed] [Google Scholar]
- 56.Maxwell K., Johnson G.N. Chlorophyll fluorescence–a practical guide. J. Exp. Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 57.Force L., Critchley C., van Rensen J.J.S. New fluorescence parameters for monitoring photosynthesis in plants. 1. The effect of illumination on the fluorescence parameters of the JIP-test. Photosynth. Res. 2003;78:17–33. doi: 10.1023/A:1026012116709. [DOI] [PubMed] [Google Scholar]
- 58.Pereira W.E., de Siqueira D.L., Martinez C.A., Puiatti M. Gas exchange and chlorophyll fluorescence in four Citrus rootstocks under aluminum stress. J. Plant Physiol. 2000;157:513–520. doi: 10.1016/S0176-1617(00)80106-6. [DOI] [Google Scholar]
- 59.Lavon R., Goldschmidt E.R., Salomon R., Frank A. Effect of potassium, magnesium, and calcium deficiencies on carbohydrate pools and metabolism in Citrus leaves. J. Am. Soc. Hortic. Sci. 1995;120:54–58. doi: 10.21273/JASHS.120.1.54. [DOI] [Google Scholar]
- 60.Shen Y., Xiao J., Yang H., Zhang S. Effects of magnesium stress on growth, distribution of several mineral elements and leaf ultrastructure of ‘Harumi’ tangor. Acta Hortiv. Sin. 2011;38:849–858. [Google Scholar]
- 61.Li Q., Chen L.-S., Jiang H.-X., Tang N., Yang L.-T., Lin Z.-H., Li Y., Yang G.-H. Effects of manganese-excess on CO2 assimilation, ribulose-1, 5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport of leaves, and antioxidant systems of leaves and roots in Citrus grandis seedlings. BMC Plant Biol. 2010;10:42. doi: 10.1186/1471-2229-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Long A., Zhang J., Yang L.-T., Ye X., Lai N.-W., Tan L.-L., Lin D., Chen L.-S. Effects of low pH on photosynthesis, related physiological parameters and nutrient profile of Citrus. Front. Plant Sci. 2017;8:185. doi: 10.3389/fpls.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang T.-Y., Cai L.-Y., Qi Y.-P., Yang L.-T., Lai N.-W., Chen L.-S. Increasing nutrient solution pH alleviated aluminum-induced inhibition of growth and impairment of photosynthetic electron transport chain in Citrus sinensis seedlings. BioMed. Res. Int. 2019;2019:9058715. doi: 10.1155/2019/9058715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo P., Li Q., Qi Y.-P., Yang L.-T., Ye X., Chen H.-H., Chen L.-S. Sulfur-mediated-alleviation of aluminum-toxicity in Citrus grandis seedlings. Int. J. Mol. Sci. 2017;18:2570. doi: 10.3390/ijms18122570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lichtenthaler H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- 66.Guo P., Qi Y.-P., Cai Y.-T., Yang T.-Y., Yang L.-T., Huang Z.-R., Chen L.-S. Aluminum effects on photosynthesis, reactive oxygen species and methylglyoxal detoxification in two Citrus species differing in aluminum tolerance. Tree Physiol. 2018;38:1548–1565. doi: 10.1093/treephys/tpy035. [DOI] [PubMed] [Google Scholar]
- 67.Chen L.-S., Cheng L. Photosystem 2 is more tolerant to high temperature in apple (Malus domestica Borkh.) leaves than in fruit peel. Photosynthetica. 2009;47:112–120. doi: 10.1007/s11099-009-0017-4. [DOI] [Google Scholar]
- 68.Banks J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practitioners. Tree Physiol. 2017;37:1128–1136. doi: 10.1093/treephys/tpx059. [DOI] [PubMed] [Google Scholar]
- 69.Sang W., Huang Z.-R., Yang L.-T., Guo P., Ye X., Chen L.-S. Effects of high toxic boron concentration on protein profiles in roots of two Citrus species differing in boron-tolerance revealed by a 2-DE based MS approach. Front. Plant Sci. 2017;8:180. doi: 10.3389/fpls.2017.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.