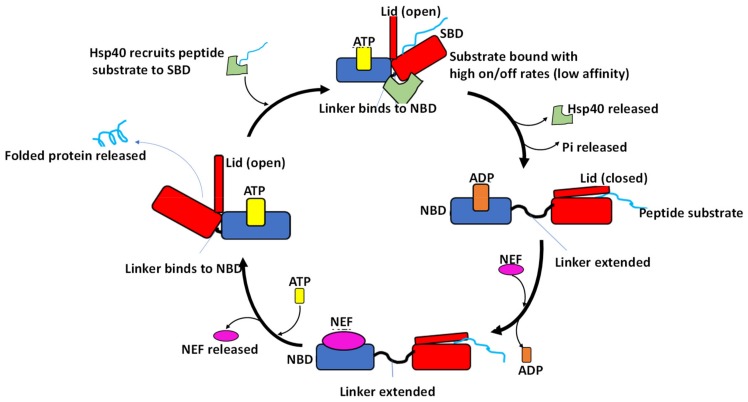
Figure 4.
Hsp70 functional cycle. The Hsp40 coupled-protein substrate is delivered to the SBD of the ATP-bound Hsp70. ATP is hydrolyzed and Hsp40 is released from the chaperone complex. Upon Hsp70 binding to ADP, its lid closes to tightly clamp the substrate within the SBD. A nucleotide exchange factor (NEF) facilitates the exchange of ADP for ATP. In the Hsp70-ATP conformation, the lid is open, and the chaperone possesses lower affinity for peptide. This results in the release of the peptide substrate from Hsp70. The fully folded protein is subsequently released. Figure adapted from [44].