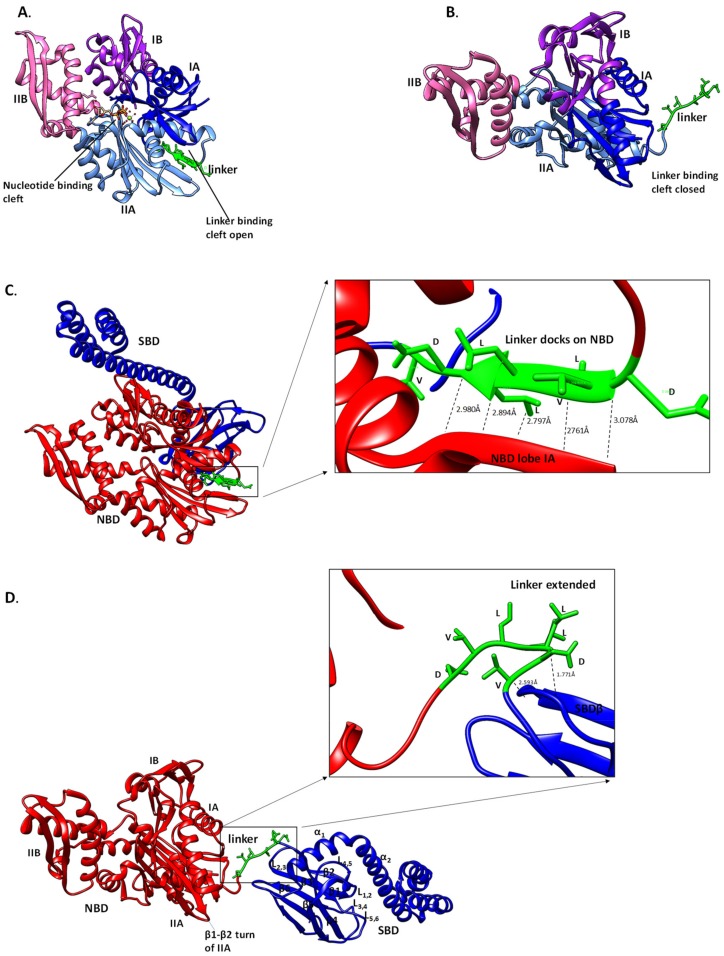
Figure 5.
Orientation of linker of Hsp70 in the presence of ATP/ADP. Hsp70 conformation is regulated variably by nucleotides. The conformational changes in the NBD upon ATP binding result in the opening of the linker leading it to docking onto the substrate binding cleft, and ultimately docking onto the NBD (A). In the ADP and apo states, the linker binding cleft at the NBD is closed and the linker assumes an extended conformation (B). Full length Hsp70 assumes a compact conformation in the ATP-bound state and the linker docks onto the NBD, forming five H bonds (C). However, the protein assumes a relaxed conformation in the ADP-bound and apo states, respectively. The linker also assumes an extended conformation forming two H bonds with the SBD (D). The templates used for generating the Hsp70 models were: full length Hsp70 in ATP bound form (4po2; [56]), in ADP-bound state (c2khoA; [57]), the NBD in ATP-bound state (c4gniA; [58]), the NBD in ADP bound state (c3iucC; [59]) and NBD in apo state (c4kboA; [60]), respectively.