Table 1.
Cross-reactivity of nanobody Nb316 with carbofuran structural analogues.
| Analogues | Molecular Structural | IC50 (ng/mL) | Cross-Reactivity (%) |
|---|---|---|---|
| Carbofuran |
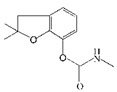
|
7.27 | 100 |
| Benfuracarb |
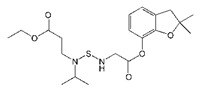
|
142.51 | 5.1 |
| Fenobucarb |
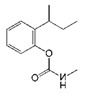
|
204.6 | 3.5 |
| Carbosulfan |
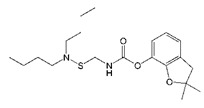
|
280.93 | 2.6 |
| 3-Hydroxycarbofuran |
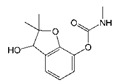
|
366.05 | 2.0 |
| Isoprocarb |
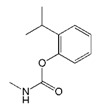
|
1351.82 | 0.5 |
| Carbaryl |
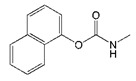
|
>2000 | <0.1 |
| Aldicarb |
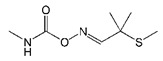
|
>2000 | <0.1 |
| Methomyl |
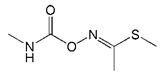
|
>2000 | <0.1 |
| Pirimicarb |
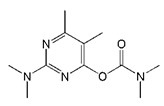
|
>2000 | <0.1 |
| Mercaptodimethur |
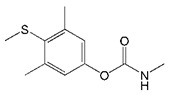
|
>2000 | <0.1 |
| Tsumacide |
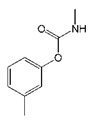
|
>2000 | <0.1 |
