Abstract
We report the observation of a strong two-photon induced fluorescence emission of Cy5-DNA within the tunable range of a Ti:Sapphire laser. The estimated two-photon cross-section for Cy5-DNA of 400 GM is about 3.5-fold higher than it was reported for rhodamine B. The fundamental anisotropies of Cy5-DNA are close to the theoretical limits of 2/5 and 4/7 for one- and two-photon excitation, respectively. We also observed an enhanced two-photon induced fluorescence (TPIF) of Cy5-DNA deposited on silver island films (SIFs). In the presence of SIFs, the TPIF is about 100-fold brighter. The brightness increase of Cy5-DNA TPIF near SIFs is mostly due to enhanced local field.
Keywords: Two-photon excitation, Cy5, Silver islands
Cyanine dyes are frequently used in genomic analysis [1,2]. The common practice is to label the cDNA pool from the experimental and control samples with two different fluorophores such as Cy3 (N,N′-(dipropyl)-tetramethylindocarbocyanine) and Cy5 (N,N′-(dipropyl)-tetramethylindodicarbocyanine) [3,4]. The relative fluorescence signals of these two dyes from each location on the DNA array correspond to the relative amount of each mRNA present in the experimental and control samples.
In this manuscript, we describe two-photon induced fluorescence of Cy3 and Cy5 labeled DNA. Two-photon excitation became a preferable tool in confocal microscopy [5–7]. This was possible because of a development of robust Ti:Sapphire femtosecond lasers. The long wavelength and localized excitation provide less unwanted scattering and less overall sample photodamage. In the last decade, many commonly used fluorophores have been characterized with multiphoton excitation [8–13]. Also, several compounds have been designed to possess large two-photon absorption cross-sections [14–18]. Two-photon excitation has been already employed with array techniques and high throughput screening (HTS) [19].
We examined Cy3- and Cy5- labeled DNA (23-mer) using Ti:Sapphire femtosecond laser within its tunable range (740–900 nm). Cy-labeled DNA was present either in a buffer solution or deposited as a monolayer on silver island films (SIFs). It is already well established that the presence of silver particles on the surface can enhance the fluorescence of deposited fluorophores by an order of magnitude or more [20–25]. This enhancement depending on fluorophore–SIF distance [26] is a result of two factors: increased local field near metal particles and increased radiative rate—the phenomenon we called radiative decay engineering (RDE) [27–29]. RDE effect is due to interaction of excited molecule with the metal particle and we do not expect its dependence on the mode of excitation. In contrast, the effect of the enhanced local field will be different for one- or two-photon excitation. We and others have already observed metal enhanced emission using two-photon excitation [30–32].
We believe that a significant enhancement of the two-photon cross-section of the Cy dyes will make them more useful for research in biotechnology and cell biology.
Materials and methods
Sample preparation
The oligonucleotides labeled with biotin, Cy3 (N,N′-(dipropyl)-tetramethylindocarbocyanine) or Cy5 (N,N′-(dipropyl)-tetramethylindodicarbocyanine) (Scheme 1) were obtained from the Biopolymer Shared Service at the University of Maryland, School of Medicine. Nanopure H2O (>18.2 MΩ), purified using Millipore Milli-Q Gradient System, was used for all experiments. Buffer components were purchased from Sigma–Aldrich (St. Louis, MO).
Scheme 1.
Structures of Cy3-DNA and Cy5-DNA.
Solutions of ds-DNA samples (Cy3-DNA or Cy5-DNA) were prepared by mixing complementary oligonucleotides in hybridization buffer (5 mM Hepes, pH 7.5, 0.1 M KCl, and 0.25 mM EDTA) to a final concentration of 1.3 μM and very slow cooling after incubation at 70 °C for 2 min.
The reference rhodamine B (RhB) sample in MeOH was used in1.3 μM concentration.
The quartz slides (Starna Cell, Atascadero, CA) used for silver island film (SIF) preparation were first cleaned overnight by soaking in 10:1 (v/v) mixture of H2SO4 (95–98%) and H2O2 (30%). After washing with ultrapure water, the quartz surface was coated with amino groups by dipping the slides in a 1% aqueous solution of 3-aminopropyltriethoxysilane (APS) for 30 min at room temperature. The slides were washed extensively with water and air-dried. SIF deposition was accomplished as described previously [23]. Briefly, 10 drops of fresh 5% NaOH solution were added to a stirring silver nitrate solution (0.25 g in 35 ml water). One milliliter of ammonium hydroxide was added drop-by-drop to redissolve the dark-brown precipitate. The solution was cooled to 5 °C in an ice bath and a fresh solution of d-glucose(0.36 g in 10 ml water) was added, followed by placing two pairs of dried quartz slides into the solution. The mixture was stirred for 2 min in ice bath and then allowed to warm up to 30 °C for the next 5 min. As the color of the mixture turned from yellow-greenish to yellow-brown, the color of the slides became greenish. The slides were removed from the beaker, rinsed with water, and bath sonicated for 1 min at room temperature. Only half of one side of each slide was coated with silver islands. The unsilvered and silvered regions of the slides were incubated overnight with 10 μM avidin (egg white, Molecular Probes).
Slides coated with SIFs and avidin monolayers were then incubated (45 min, room temperature) with 2 μM solution of double-stranded Cy5-DNA-biotin, which was prepared by mixing single-stranded oligos (one oligo labeled with Cy5 dye and second, complementary labeled with biotin on 5′ end) in hybridization buffer (5 mM Hepes, pH 7.5, 0.1 M KCl, and 0.25 mM EDTA). After washing with the same buffer, each slide was assembled with the grooved part of 0.5 mm demountable cuvette and filled with buffer.
Fluorescence measurements
One-photon excitation.
Fluorescence spectra with one-photon excitation were measured using SLM 8000 spectrofluorometer. The lifetimes of Cy5-DNA were measured with 10 GHz frequency-domain fluorometer [33]. The excitation at 635 nm was from DCM dye laser. Cavity-dumped dye laser provided 7 ps pulses with a repetition of 3.86 MHz. Intensity decays were analyzed by nonlinear least squares in terms of multiexponential model.
Two-photon excitation.
The steady state and intensity decay measurements of two-photon induced fluorescence were performed using lock-to-clock Ti:Sapphire laser, as described in [30,34,35].
Results and discussion
Two-photon excitation in solution
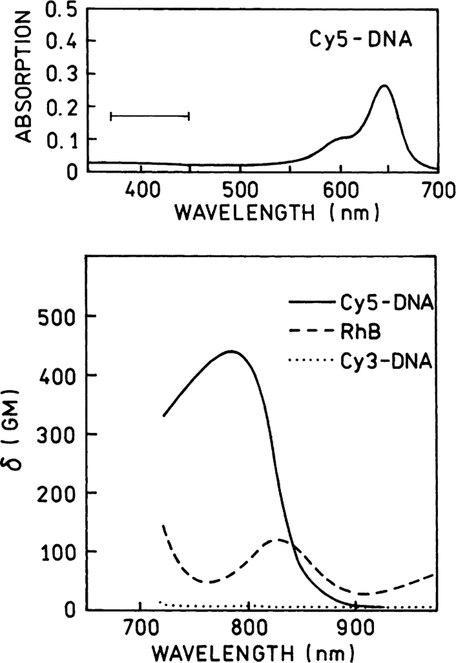
First, we measured the emission spectra of equimolar(1.3 μM) solutions of Cy3-DNA, Cy5-DNA in hybridization buffer and RhB in methanol (a reference with known two-photon cross-section [15]) using different excitation wavelengths from Ti:Sapphire laser. At each wavelength the conditions were steady while the emission spectra of all three compounds were recorded. The selected spectra are shown in Fig. 1. At longer wavelengths, above 870 nm there are very little two-photon induced emissions from Cy3- or Cy5-DNA, compared to RhB in methanol. However, at shorter wavelengths the Cy5-DNA shows an unexpected strong TPIF. Below 820 nm excitation the brightness of the Cy5-DNA sample exceeds that of the RhB sample. However, the Cy3-DNA does not show a significant TPIF within the entire wavelength region, 740–900 nm. Using known values of RhB cross-sections [15] we calculated two-photon absorption cross-section for Cy3-DNA and Cy5-DNA. For these calculations we took the quantum efficiencies—0.24, 0.20, and 0.84 [36] for Cy3-DNA, Cy5-DNA, and RhB, respectively. The Cy5-DNA displays 3–4-fold higher two-photon cross-section than RhB (Fig. 2, bottom). This was a pleasant surprise because RhB is known as one of the best two-photon absorbers. Such strong two-photon absorptivity indicates an allowed two-photon transition with energy corresponding to 400 nm. It is difficult to predict an effective two-photon excitation because there is no such transition in the one-photon absorption spectrum (Fig. 2, top).
Fig. 1.
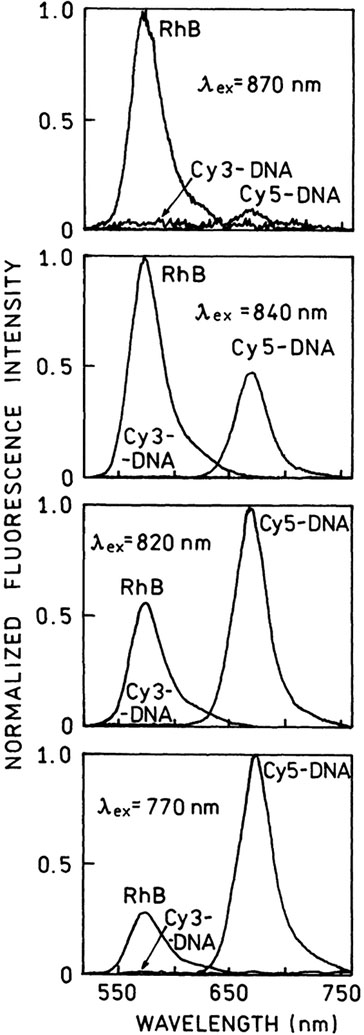
Emission spectra of two-photon induced fluorescence of Cy3-DNA and Cy5-DNA in hybridization buffer (5 mM Hepes, pH 7.5,0.1 M KCl, and 0.25 mM EDTA) and RhB in methanol. The concentrations of all three fluorophores were the same, 1.3 μM. The experimental conditions were kept constant during the measurements. The excitation was from femtosecond, tunable Ti:Sapphire laser.
Fig. 2.
The two-photon cross-sections for Cy3-DNA (⋯) and Cy5-DNA (—) in hybridization buffer calculated using RhB in methanol (- - -) as a reference [36]. The upper panel shows the one-photon absorption spectrum of Cy5-DNA. The horizontal bar marks the energy level of observed two-photon transition. This transition is not seen in the one-photon spectrum.
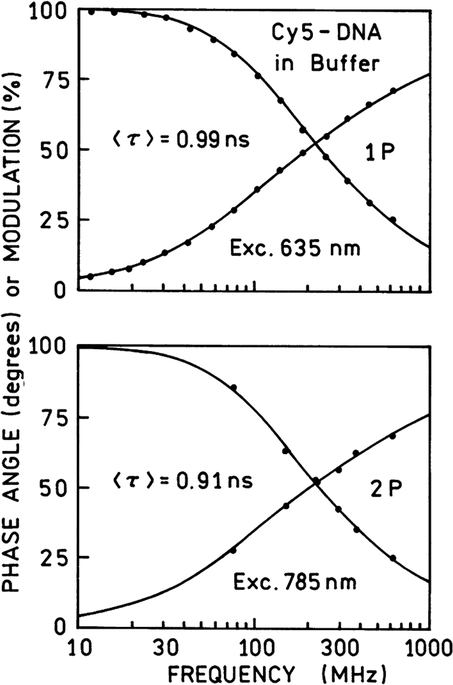
The lifetimes measured with one- and two-photon excitation are similar (Fig. 3), indicating that the fluorescence occurred from the same excited electronic state. This was not a surprise since we observed such an agreement in many multiphoton studies [10].
Fig. 3.
Frequency-domain intensity decays of Cy5-DNA in hybridization buffer with one-photon (top) and two-photon (bottom) excitation. The lifetimes are similar for one- and two-photon excitation, indicating that emission occurs from the same fluorescent state.
There are two fluorescence parameters different for one- and two-photon excitation: (1) the excitation power dependence on emission and (2) fluorescence anisotropy. Before collecting any TPIF data we checked a square dependence of observed fluorescence signal on the excitation power. The attenuation of the excitation power 2.23-fold (0.4 OD neutral density filter) resulted in a 4.9 decrease of fluorescence signal which proves that we observed a two-photon process.
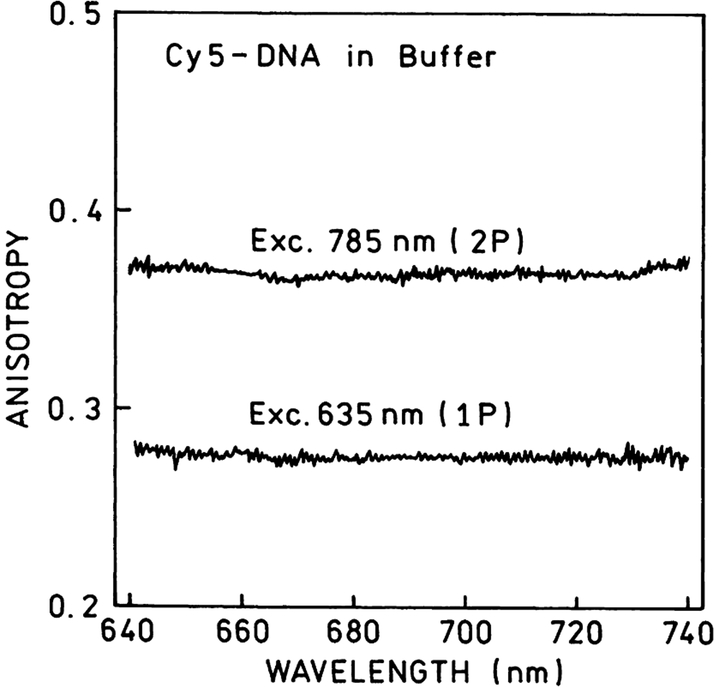
The anisotropies observed with one- and two-photon excitation are different. The emission anisotropy spectra of Cy5-DNA solution are presented in Fig. 4. TPIF anisotropies are higher approximately by factor 10/7 than those observed with one-photon excitation. This is the result of higher photoselection induced by two-photon absorption [37–40]. The high values of observed anisotropies in solution indicate that attached to the DNA fluorophore cannot rotate freely.
Fig. 4.
Emission anisotropy spectra for Cy5-DNA in buffer solution obtained with one- and two-photon excitation. The high anisotropy values indicate a low mobility of Cy5-DNA molecules. The ratio of two-photon to one-photon anisotropies is very close to 10/7, the theoretical value for parallel transitions.
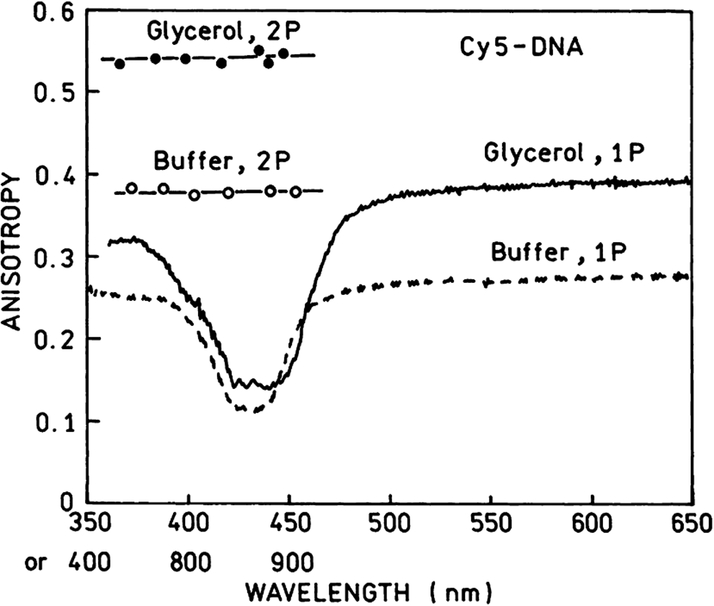
The excitation anisotropy spectra of Cy5-DNA are shown in Fig. 5. In Fig. 5 are also presented anisotropy spectra of Cy5-DNA in 90% glycerol at −5°C. The measured anisotropies for one- and two-photon excitation in rigid solution are very close to fundamental values of 2/5 and 4/7 for one- and two-photon photoselections.
Fig. 5.
The excitation anisotropy spectra of Cy5-DNA in buffer solution at 20°C and in 90% glycerol at −5°C. The anisotropies in glycerol are close to the maximal fundamental values 0.4 for one-photon and 0.57 for two-photon excitation. There is a weak one-photon transition around 430 nm with low anisotropy.
Two-photon excitation on SIFs
We have already reported fluorescence measurements of Cy3- and Cy5-DNA monolayers deposited on quartz and silvered surfaces with one-photon excitation [23,26]. We noticed about a 10-fold increase of brightness on SIFs with a simultaneous decrease of lifetime compared to the unsilvered surface.
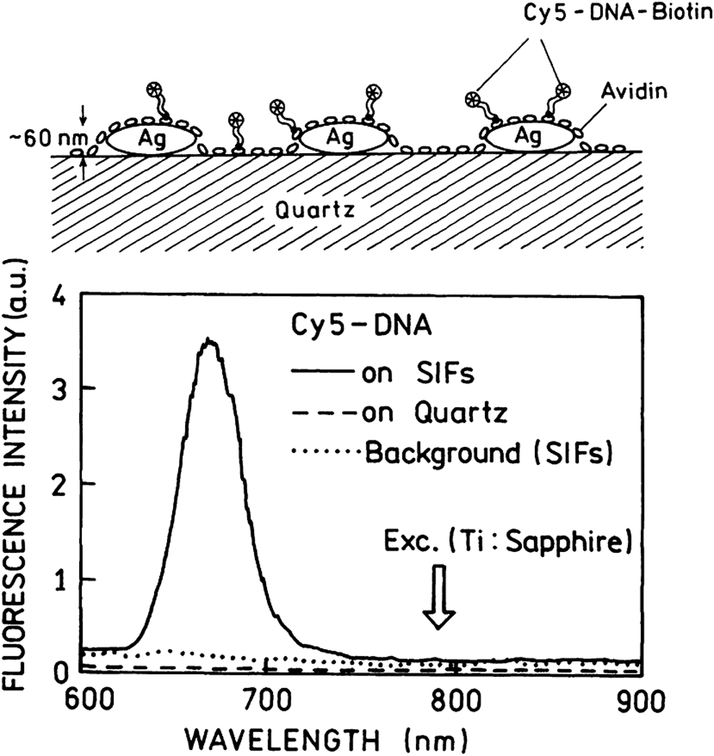
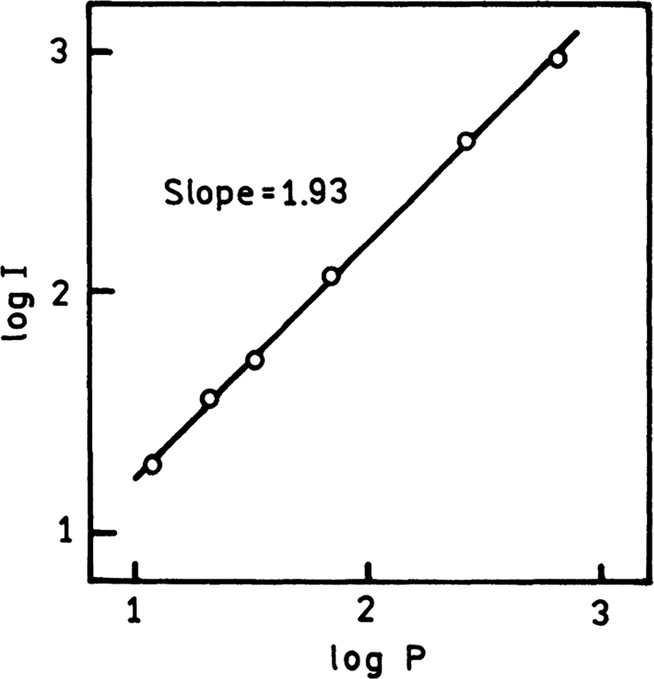
TPIF spectra of Cy5-DNA monolayer on SIFs and unsilvered quartz substrate are presented in Fig. 6. We attempted to estimate how many times the TPIF is more effective on SIFs compared to quartz only surface. The silvered part of the slide was about 90–100-fold brighter than unsilvered upon the same excitation illumination. We were aware of SIFs emission background. Silver and gold nanostructures are known to be photoactivated with light and emit characteristic “blinking” [41–44]. The background from SIFs, although stronger than Cy5-DNA emission on quartz, was significantly lower than Cy5 TPIF signal from the sample. We examined the Cy5-DNA TPIF dependence on the laser power and obtained almost ideal square dependence (Fig. 7).
Fig. 6.
Two-photon-induced emission spectra of Cy5-DNA on SIFs and quartz. The brightness of Cy5-DNA on SIFs is approximately 100-fold higher than on quartz. In the top panel is shown the schematic of Cy5-dye deposited on SIFs.
Fig. 7.
The excitation power dependence of two-photon induced fluorescence of Cy5-DNA on SIFs. The excitation was 785 nm and emission was observed at 665 nm.
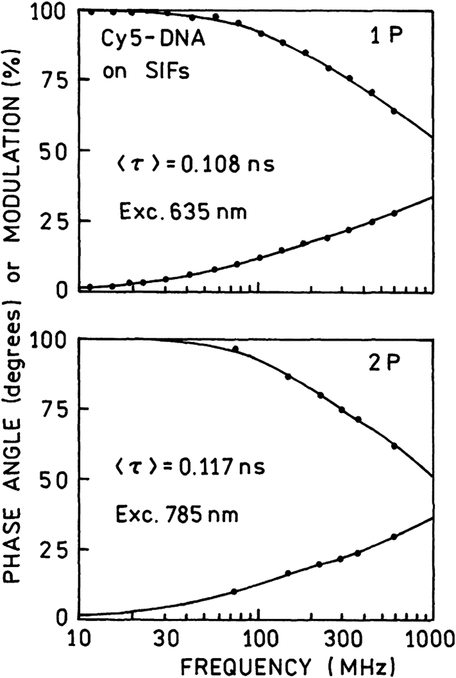
We wondered if the stronger enhancement of Cy5-DNA TPIF on SIFs compared to that with one-photon excitation is accompanied by further decrease of lifetime. Fig. 8 shows the intensity decays of Cy5-DNA on SIFs observed with one-photon (top) and two-photon excitation (bottom). These decays are very similar (Table 1), indicating that a dominant portion of the brightness increase with two-photon excitation is due to a stronger local field. The rate of the two-photon absorption depends on the square of the excitation light field.
Fig. 8.
Frequency-domain intensity decays of Cy5-DNA on SIFs observed with one-photon (top) and two-photon (bottom) excitation. The lifetimes are significantly shorter than observed in solution or on quartz (Table 1).
Table 1.
Multiexponential analysis of Cy5-DNA intensity decays
| Conditions | <τ> (ns) | (ns) | α1 | τ1 (ns) | α2 | τ2 (ns) | α3 | τ3 [ns] | |
|---|---|---|---|---|---|---|---|---|---|
| Buffer | |||||||||
| 1P | 0.99a | 1.23b | 0.466 | 0.47 | 0.534 | 1.44 | — | — | 1.0 |
| 2P | 0.91 | 1.20 | 0.498 | 0.40 | 0.502 | 1.42 | — | — | 1.3 |
| SIFs | |||||||||
| 1P | 0.108 | 0.401 | 0.817 | 0.049 | 0.157 | 0.24 | 0.026 | 1.10 | 0.9 |
| 2P | 0.117 | 0.418 | 0.754 | 0.050 | 0.208 | 0.2 | 0.038 | 1.02 | 1.4 |
Conclusions
We believe that the observation of very strong two-photon transition of Cy5 within a convenient Ti:Sapphire laser excitation is an important finding. The use of effective two-photon excitation with DNA arrays or HTS microplates can be beneficial because of lower background and minimal volume needed for measurements with a focused excitation.
The combination of TPIF and fluorescence enhancement on SIFs or other metallic structures results in at least two order of magnitude increased brightness which improves signal/noise ratio and potentially enables decrease of used dye concentrations. These properties can be especially useful in such fluorescence techniques as correlation spectroscopy or single molecule detection (SMD).
Acknowledgments
This research was supported by a grant from NIH, NCRR, RR-08119. Z.G. thanks Philip Morris USA, for financial support.
References
- [1].Deyholos MK, Galbraith DV, High-density microarrays for gene expression analysis, Cytometry 43 (2001) 229–239. [PubMed] [Google Scholar]
- [2].Harrington CA, Rosenow C, Retief J, Monitoring gene expression using DNA microarrays, Curr. Opin. Microbiol 3(3) (2000) 285–291. [DOI] [PubMed] [Google Scholar]
- [3].Brown PO, Botstein D, Exploring the new world of the genome with DNA microarrays, Nat. Genet. Suppl 21 (1999) 33–37. [DOI] [PubMed] [Google Scholar]
- [4].Schena M, Heller RA, Theriault TP, Konrad K, Lachenmeier E, Davis RW, Microarrays: biotechnology’s discovery platform for functional genomics, Trends Biotechnol 16 (1998) 301–306. [DOI] [PubMed] [Google Scholar]
- [5].Denk W, W Piston D, Webb WW, Two-photon molecular excitation in laser-scanning microscopy, in: Powley JB (Ed.), Handbook of Biological Confocal Microscopy, second ed., Plenum Press, New York, 1995, pp. 445–458. [Google Scholar]
- [6].Hell SW (guest ed.), Special issue on nonlinear optical microscopy, Bioimaging 4, (1996). [Google Scholar]
- [7].Diaspro A, Introduction to two-photon microscopy, Microsc. Res. Tech 47 (1999) 163–164. [DOI] [PubMed] [Google Scholar]
- [8].Gryczynski I, Szmacinski H, Lakowicz JR, On the possibility of calcium imaging using indo-1 with three-photon excitation, Photochem. Photobiol 62 (4) (1995) 804–808. [DOI] [PubMed] [Google Scholar]
- [9].Lakowicz JR, Gryczynski I, Malak H, Schrader M, Engelhardt P, Kano H, Hell SW, Time-resolved fluorescence spectroscopy and imaging of DNA labeled with DAPI and Hoechst 33342 using three-photon excitation, Biophys. J 72 (1997) 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lakowicz JR, Gryczynski I, Multiphoton excitation of biochemical fluorophores, in: Lakowicz JR (Ed.), Topics in Fluorescence Spectroscopy, Nonlinear and two-photon-induced fluorescence, vol. 5, Plenum Press, New York, 1997, pp. 87–144. [Google Scholar]
- [11].Lakowicz JR, Gryczynski I, Piszczek G, Murphy CJ, Emission spectral properties of cadmium sulfide nanoparticles with multiphoton excitation, J. Phys. Chem. B 106 (1999) 5365–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lakowicz JR, Castellano FN, Gryczynski I, Gryczynski Z, Dattelbaum JD, Two-photon excitation of rhenium metal–ligand complexes, J. Photochem. Photobiol. A Chem 122 (1999) 95–101. [Google Scholar]
- [13].Fischer A, Cremer C, Stelzer EHK, Fluorescence of coumarins and xanthenes after two-photon absorption with a pulsed titanium-sapphire laser, Appl. Opt 34 (12) (1995) 1989–2003. [DOI] [PubMed] [Google Scholar]
- [14].Chung SJ, Maciel GS, Pudavar HE, Lin TC, He GS, Swiatkiewicz J, Prasad PN, Two-photon properties and excitation dynamics of poly(p-phenylenevinylene) derivatives carrying phenylanthracene and branched alkoxy pendents, J. Phys. Chem. A 106 (2002) 7512–7520. [Google Scholar]
- [15].Xu C, Webb WW, Multiphon excitation of molecular fluorophores and nonlinear laser microscopy, in: Lakowicz JR (Ed.), Topics in Fluorescence Spectroscopy, Vol. 5: Nonlinear and two photon-induced fluorescence, Plenum Press, New York, 1997, pp. 471–540. [Google Scholar]
- [16].Ohulchanskyy TY, Pudavar HE, Yarmoluk SM, Yashchuk VM, Bergey EJ, Prasad PN, A monomethine cyanine dye Cyan 40 for two-photon-excited fluorescence detection of nucleic acids and their visualization in live cells, Photochem. Photobiol 77 (2) (2003) 138–145. [DOI] [PubMed] [Google Scholar]
- [17].He GS, Lin T-C, Prasad PN, Kannan R, Vaia RA, Tan L-S, Study of two-photon absorption spectral property of a novel nonlinear optical chromophore using femtosecond continuum, J. Phys. Chem. B 106 (2002) 11081–11084. [Google Scholar]
- [18].Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, Webb WW, Water-soluble quantum dots for multiphoton fluorescence imaging in vivo, Science 300 (2003) 1434–1436. [DOI] [PubMed] [Google Scholar]
- [19].Lakowicz JR, Gryczynski I, Gryczynski Z, High throughput screening with multiphoton excitation, J. Biomol. Screen 4 (6) (1999) 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tarcha PJ, De Saja-Gonzales J, Rodriques-Llorente S, Aroca R, Surface-enhanced fluorescence on SiO2 coated silver island films, Appl. Spectrosc 53 (1999) 43–48. [Google Scholar]
- [21].German AE, Gachko GA, Dependence of the amplification of giant Raman scattering and fluorescence on the distance between an adsorbed molecule and a metal surface, J. Appl. Spectrosc 68 (2001) 987–992. [Google Scholar]
- [22].Sokolov K, Chumanov G, Cotton TM, Enhancement of molecular fluorescence near the surface of colloidal metal films, Anal. Chem 70 (1998) 3898–3905. [DOI] [PubMed] [Google Scholar]
- [23].Malicka J, Gryczynski I, Fang J, Lakowicz JR, Fluorescence spectral properties of cyanine dye-labeled DNA oligomers on surfaces coated with silver particles, Anal. Biochem 317 (2003) 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lakowicz JR, Malicka J, D’Auria S, Gryczynski I, Release of the self-quenching of fluorescence near silver metallic surfaces, Anal. Biochem 320 (2003) 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Malicka J, Gryczynski I, Lakowicz JR, Enhanced emission of highly labeled DNA oligomers near silver metallic surfaces, Anal. Chem 75 (17) (2003) 4408–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR, Effects of fluorophore-to-silver distance on the emission of cyanine-dye-labeled oligonucleotides, Anal. Biochem 315 (2003) 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lakowicz JR, Radiative decay engineering: biophysical and biomedical applications, Anal. Biochem 298 (2001) 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lakowicz JR, Shen Y, D’Auria S, Malicka J, Gryczynski Z, Gryczynski I, Radiative decay engineering, 2. Effects on silver island films on fluorescence intensity, lifetimes and resonance energy transfer, Anal. Biochem 301 (2002) 261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lakowicz JR, Malicka J, Gryczynski I, Gryczynski Z, Geddes CD, Radiative decay engineering: the role of photonic mode density in biotechnology, J. Phys. D Appl. Phys 36 (2003) R240–R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gryczynski I, Malicka J, Shen Y, Gryczynski Z, Lakowicz JR, Multiphoton excitation of fluorescence near metallic particles: enhanced and localized excitation, J. Phys. Chem. B 106 (2002) 2191–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wenseleers W, Stellacci F, Meyer-Friedrichsen T, Mangel T, Bauer CA, Pond SJK, Marder SR, Perry JW, Five orders-of-magnitude enhancement of two-photon absorption for dyes on silver nanoparticle fractal clusters, J. Phys. Chem. B 106 (2002) 6853–6863. [Google Scholar]
- [32].Maliwal BP, Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR, Fluorescence properties of labeled proteins near silver colloid surfaces, Biopolymers 70 (2003) 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Laczko G, Gryczynski I, Gryczynski Z, Wiczk W, Malak H, Lakowicz JR, A 10-GHz frequency-domain fluorometer, Rev. Sci. Instrum 61 (1990) 2331–2337. [Google Scholar]
- [34].Castellano FN, Malak H, Gryczynski I, Lakowicz JR, Creation of metal-to-ligand charge transfer excited states with two-photon excitation, Inorg. Chem 36 (24) (1997) 5548–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Malak H, Castellano FN, Gryczynski I, Lakowicz JR, Two-photon excitation of ethidium bromide labeled DNA, Biophys. Chem 67 (1997) 35–41. [DOI] [PubMed] [Google Scholar]
- [36].Malicka J, Gryczynski I, Kusba J, Lakowicz JR, Effects of metallic silver island films on resonance energy transfer between N,N′-(dipropyl)-tetramethylindocarbocyanine (Cy3) and N,N′-(dipropyl)-tetramethylindodicarbocyanine (Cy5)-labeled DNA, Biopolymers 70 (2003) 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lakowicz JR, Gryczynski I, Gryczynski Z, Danielsen E, Wirth MJ, Time resolved fluorescence intensity and anisotropy decays of 2,5-diphenyloxazole by two-photon excitation and frequency-domain fluorometry, J. Phys. Chem 98 (1992) 3000–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Callis PR, On the theory of two-photon induced fluorescence anisotropy with applications to indoles, J. Chem. Phys 99 (1993) 27–37. [Google Scholar]
- [39].Chen S-Y, van der Meer BW, Theory of two-photon induced fluorescence anisotropy decay in membranes, Biophys. J 64 (1993) 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wan C, Johnson CK, Time-resolved anisotropic two-photon spectroscopy, Chem. Phys 179 (1994) 513–531. [Google Scholar]
- [41].Peyser LA, Vinson AE, Bartko AP, Dickson RM, Photoactivated fluorescence from individual silver nanoclusters, Science 291 (2001) 103–106. [DOI] [PubMed] [Google Scholar]
- [42].Peyser LA, Lee T-H, Dickson RM, Mechanism of Agn nanocluster photoproduction from silver oxide films, J. Phys. Chem. B 106 (2002) 7725–7728. [Google Scholar]
- [43].Geddes CD, Parfenov A, Gryczynski I, Lakowicz JR, Luminescent blinking from silver nanostructures, J. Phys. Chem. B 107 (2003) 9989–9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Geddes CD, Parfenov A, Gryczynski I, Lakowicz JR, Luminescent blinking of gold nanoparticles, Chem. Phys. Lett 380 (2003) 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]