ABSTRACT
Background: Melanoma of unknown primary (MUP) is an uncommon clinical subtype of melanoma of known primary (MKP).
Objectives: We aimed to compare treatment outcomes of MUP and MKP patients who had undergone therapy with immune checkpoint inhibitors (ICPI).
Methods: We studied 41 metastatic melanoma patients (32 with MKP and 9 with MUP) with an indication for ICPI.
Results: Clinical characteristics such as age, gender, stage of disease, etc., did not significantly differ (P < .05) between MUP and MKP patients. 20/32 (62.5%) melanoma-specific deaths (MSD) were observed in the MKP group, whereas 2/9 (22.2%) were detected in the MUP group (P = .035). On logistic regression, the MUP status proved to be an independent predictor for a more favorable outcome under immunotherapy when compared to MKP (P = .030).
Conclusion: Our preliminary results indicate that MUP patients show better clinical outcome under ICPI when compared to MKP.
KEYWORDS: Melanoma of unknown primary, immunotherapy, ipilimumab, nivolumab, pembrolizumab
Introduction
Melanoma accounts for about three-quarters of skin-cancer-related mortality as indicated by its ability to metastasize very early.1,2 Once melanoma has spread into the lymph nodes, or even visceral organs and the brain, it remains a life-threatening disease despite the advent of molecular targeted or immunotherapies.1,2 Melanoma of unknown primary (MUP)3–17 represents an unusual subtype of melanoma which was reported to be about 2–9% of melanoma of known primary (MKP) at all stages.3
Some of the literature in the different geographic regions demonstrated better overall survival of MUP than of MKP.15 Other publications, however, reported an equal or even worse clinical outcome for patients with MUP when compared to MKP with comparable stage of disease.3–17 Hence, the prognosis of patients with MUP compared to MKP patients is still unclear and likely depends on the clinical scenario investigated. A 5-year overall survival rate of MUP before the era of the immune checkpoint inhibitor was reported to range from 8% to 18% with the most common metastatic sites in lymph nodes and gastrointestinal tract.15 Many hypotheses were documented in relation to the etiology of MUP. Accordingly, the occurrence of MUP may be due to immune-mediated total regression of the primary tumor; alternatively, in MUP patients, melanoma may not be metastatic but may originate de-novo from nevus cell aggregates in lymph nodes, for example.15
If immune-mediated mechanisms really can result in the development of MUP, immunotherapies might be a reasonable approach for patients with this uncommon melanoma subtype. Immune checkpoint (ICP) modulating agents such as the cytotoxic T-lymphocyte-associate protein 4 (CTLA-4) blocker ipilimumab and the programmed death 1 (PD-1) blocking antibodies nivolumab and pembrolizumab, turned out to be effective in the treatment of stage IV and as well stage III melanoma.1,5 However, there is a lack of data regarding the efficacy of ICP inhibitors (ICPI) in patients with MUP. In the present pilot study we aimed to assess whether MUP patients have better outcomes under ICPI than patients with MKP.
Methods
Patients
This study was performed at the Skin Cancer Center of the Ruhr-University Bochum (Bochum, Germany) after having been approved by the local ethics review board of the Ruhr-University Bochum. We searched our databases for patients with metastatic MUP and MKP who underwent immunotherapy between the year 2014 to 2018 including mono-pembrolizumab, mono-nivolumab and nivolumab plus ipilimumab. We only included patients with sufficient follow-up data receiving at least three cycles of immunotherapy. The initial diagnosis of MUP was made based on the patient's history and clinical signs consistent with metastatic disease, along with histopathology of a tissue specimen that confirmed the presence of malignant melanocytes, such as biopsy of a lymph node or needle core biopsy of a solid organ metastasis. The histological features of MUP on a tissue specimen included: Positive melanocytic immunohistochemical markers, such as S100B, Melan-A/MART, and/or HMB-45. In all cases, an initial complete work-up was performed including imaging staging with computed tomography (CT) of the chest, abdomen and pelvis, cranial magnetic resonance imaging, and in some cases PET-CT imaging as well. According to Dasgupta et al.18, the following exclusion criteria for MUP diagnosis were applied: 1) evidence of previous orbital exenteration or enucleation; 2) evidence of previous skin excision, electrodessication, cauterization or other surgical manipulation of a mole, freckle, birthmark, paronychia or skin blemish; 3) evidence of metastatic melanoma in a draining lymph node with a scar in the area of skin supplying that lymph node basin; 4) lack of a non-thorough physical examination, including the absence of an ophthalmologic, anal and genital examination.18
Treatment and monitoring
Ipilimumab plus nivolumab, mono-nivolumab, and mono-pembrolizumab treatments were routinely carried out in-label.1,2 Due to the high toxicity, ipilimumab plus nivolumab therapy was predominantly prescribed in younger patients with very good performance status. Data were collected with respect to patient characteristics [sex, age, disease stage, medication, adverse events (AEs), etc.] and pre-treatment lab investigations including BRAF mutation analysis (cobas® 4800 BRAF V600 Mutation Test, Roche, Grenzach-Whylen, Germany). Blood parameters such as neutrophils, lymphocytes, S100B and lactate dehydrogenase (LDH) were routinely measured. Complete work-up was regularly performed including lymph node ultrasound, thoracic and abdominal computed tomography, and cranial magnetic resonance tomography.19 Before and during immunotherapy the patients were clinically monitored as recommended by Kähler and coauthors.20 Imaging methods were routinely performed every three months.
Statistics
Data analysis was performed using the statistical package MedCalc Software (Ostende, Belgium). Distribution of data was assessed by the D`Agostino-Pearson test. Non-normally distributed data were expressed as medians and range, normally distributed as mean±SD. Data were analyzed using the independent t-test, Mann–Whitney test, Chi2 or Fisher exact test, and Kaplan–Meier curves including the log-rank test (univariable testing). Moreover, multivariable logistic regression analysis was generated including variables that were significantly associated with melanoma-specific death (MSD). The latter was defined by the time in months from the first immunotherapy dose to the clinical event. Survival curves were calculated from the time of diagnosis of primary melanoma and considered censored for non-melanoma-related deaths and unavailable data. P-values of <0.05 were considered significant.
Results
Almost all patients started and continued ICPI therapy with nivolumab (in part in combination with ipilimumab) or pembrolizumab (Table 1), except for two patients starting with 4 cycles ipilimumab following during the course of disease with over 25 and 24 cycles of pembrolizumab monotherapy, respectively. Moreover, one patient with MUP had 7 cycles of nivolumab followed by 1 cycle ipilimumab which was discontinued because of recalcitrant diarrhea.
Table 1.
Overview of tumor characteristics and clinical outcome of patients with melanoma of known primary (MKP) and melanoma of unknown primary (MUP) who underwent systemic immunotherapy with immune checkpoint inhibitors (ICPI).
| Parameters | Patients with MKP | Patients with MUP | P-value Chi2, Fisher exact, or Mann-Whitney test (univariable analysis) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age (years) mean±SD |
64.5 ± 14.3 | 71.6 ± 11.3 | = 0.50 | ||||||||||
|
Gender Female/male |
10/22 | 3/6 | = 0.92 | ||||||||||
|
Blood parameters prior to ICPI S100B (µg/l; < 0.2) LDH (U/l; 134–214) Neutrophils (1800 – 7200/µl) Lymphocytes (1000 – 4500/µl) Neutrophil/Lymphocyte ratio |
0.17 (0.05–7,9) 197 (136–415) 4696 (1651 − 10231) 1759 (550–2967) 3.1 (0.98–7.50) |
0.2 (0.03–2.5) 203 (134–458) 5523 (3838–5912) 1518 (688–3330) 3.4 (1.80–6.22) |
= 0.83 = 0.97 = 0.51 = 0.55 = 0.34 | ||||||||||
|
BRAF mutation No/yes |
26/6 | 8/1 | = 0.58 | ||||||||||
|
Sub-stages Number of patients |
IIIc | IIId | M1a | M1b | M1c | M1d | IIIc | IIId | M1a | M1b | M1c | M1d | n.a. |
| 2 | 1 | 4 | 9 | 14 | 2 | 0 | 0 | 3 | 2 | 3 | 1 | ||
|
Median number of anti-PD1 cycles Pembrolizumab/Nivolumab/Ipilimumab& Adverse events (any grade) No/yes |
10.5 (2–42) 6/15/11 patients 20/12 |
10 (5–56) 5/4/0 patients 6/3 |
= 0.27 n.a. = 0.82 |
||||||||||
|
Response and/or stable disease No/yes Melanoma-specific deaths |
19/13 12/20 |
2/7 7/2 |
= 0.052§ = 0.035 |
||||||||||
*, lactate dehydrogenase; &, combination regimen plus nivolumab; n.a., not applicable; §, trend for statistical significance.
Melanoma sub-stages of MUP and MKP patients at the time of immunotherapy initiation did not substantially differ (Table 1). Almost all patients in both groups received immunotherapy as first-line treatment. As also shown in Table 1, clinical data such as age, gender, blood parameters (LDH, S100B, neutrophils, lymphocytes, etc.), BRAF mutation status, immunotherapy cycles, and adverse events did not statistically significantly differ (P < .05) between MUP and MKP patients. No drug-related deaths were observed. The median follow-up was 24 months (3–54 months) for MUP patients and 12.5 (3–42) months for MKP patients (P = .27). Total median follow-up was 13 (3–54) months. Immunotherapy response and/or stable disease were observed in 13/32 (40.6%) MKP patients when compared to 7/9 (77.8%) patients with MUP. However, the difference between the aforementioned proportions reached only a trend for statistical significance as indicated by a P-value of 0.052. As demonstrated in Table 1, 20/32 (62.5%) MSD were observed in the MKP group, whereas 2/9 (22.2%) were detected in the MUP group. Univariable statistics revealed that significantly less MSD were observed in MUP patients when compared to MKP patients (P = .035). Apart from the MKP status MSD was also significantly associated with elevated LDH (P = .030) and S100B (P = .018). The latter parameters were included in multivariable analysis using logistic regression. The MUP status proved to be a significant independent predictor for more favorable melanoma-specific survival under immunotherapy when compared to MKP patients (P = .030), whereas elevated S100B was a significant independent predictor for MSD (P = .032). Accordingly, Kaplan–Meier curves in Figure 1 demonstrate a significantly better outcome for MUP patients with respect to MSD as indicated by a hazard ratio of 0.29 (95% CI 0.11 to 0.75; log rank test, P = .011).
Figure 1.
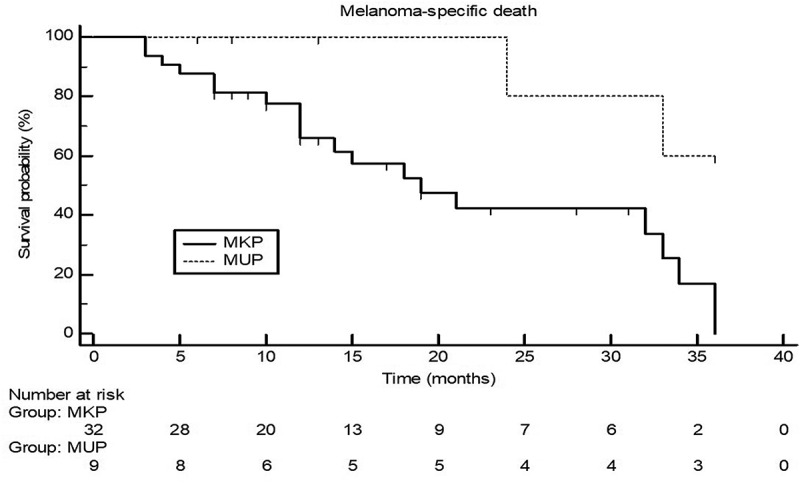
Kaplan–Meier curves for 3-year melanoma-specific death in patients with melanoma of unknown primary (MUP) and melanoma with known primary MKP) who underwent treatment with immune checkpoint inhibitors. Log-rank test was statistically significant (P = .0112) with a hazard ratio for MUP patients of 0.29 (95% confidence interval of 0.11 to 0.75).
Discussion
MUP may have a different biology to MKP, but clinical trials of novel therapies, including ICPI or BRAF/MEK inhibitors, have not reported the outcomes in this population separately.16 Utter et al.6 recently searched the New York University (NYU)’s prospective melanoma database for MUP patients treated with systemic therapy. Moreover, they searched PubMed and Google Scholar for MUP patients treated with immunotherapy or targeted therapy reported in the literature, and their response and survival data were compared to the MUP patient data from NYU.6 Both groups’ response data were finally compared to those reported for MKP. All in all, 23 NYU MUP patients received immunotherapy, including 19 patients with ipilimumab only, 1 patient with ipilimumab and nivolumab combination treatment, 2 with pembrolizumab monotherapy, and 1 with pembrolizumab followed by ipilimumab.6 The follow-up time for these patients ranged from 1 to 96 months. Three papers taken from the literature reported sufficient data on a total of 24 MUP patients treated with immunotherapy.6 Of the 24 MUP patients identified, 13 (54%) were enrolled in a phase II clinical trial assessing the efficacy of ipilimumab in pre-treated stage IV patients, 10 patients (42%) were enrolled in the ipilimumab expanded access program, and 1 patient (4%) (L-I24) was featured in a case report. Utter et al.6 reported that both the NYU MUP patients and those MUP patients described in the literature had a worse outcome on immunotherapy when compared to the general melanoma distribution treated with immunotherapy in clinical trials.6
Similarly, Verver et al.16 evaluated data for stage III or IV MUP patients extracted from a nationwide database for the period 2003–2016. They divided the population into pre- (2003–2010) and post- (2011–2016) novel therapy eras.16 In total, 2028/65.110 (3.1%) patients were diagnosed with MUP. Metastatic sites were known in 1919 of 2028 patients, and most had stage IV disease (53.8%).16 For patients with stage III MUP, the 5-year overall survival rates did not significantly differ between the pre- and post-novel eras (P = .95).16 For those with stage IV MUP, the median overall survival times were unchanged in the pre-novel era and post-novel era when novel treatments were not employed.16 Notably, overall survival significantly (P < .001) improved to 11 months when novel treatments (e.g., ICPI) were used in the post-novel era. Though Verver et al.16 did not systematically evaluated outcome differences between MKP and MUP, they observed an improved clinically outcome for MUP patients treated with novel therapies including immunotherapy. Since MUP and MKP have similarly high mutational tumor loads immunotherapies should be an effective treatment option for MUP patients as well.4,12
In the present study, we have demonstrated that MUP patients in stage IV treated with ICPI experienced significantly less MSD when compared to stage IV or unresectable stage III MKP patients. Due to the small sample size, we likely observed only a statistical trend for better response to ICPI treatment in patients with MUP as compared to patients with MKP. With regard to potentially important clinical outcome parameters, including age, gender, BRAF mutation status, treatment cycles, and adverse events, we did not observe significant differences between MKP and MUP patients, except for the relatively unbalanced use of immunotherapy combination in the MKP and MUP populations. Because of the higher toxicities frequently observed in combination immunotherapy we predominantly used ipilimumab plus nivolumab in younger patients with excellent performance status. Crucial differences between both groups with respect to the metastatic load are unlikely given the indifferent N- and M-sub-stages, LDH, and S100B levels in MUP and MKP patients evaluated in the present study. Moreover, recently established biomarkers, including baseline neutrophil/lymphocyte ratio, were not significantly different in our MKP and MUP patients.21,22 Hence, the observed survival advantage observed for MUP patients under ICPI therapy was unlikely due to a disbalance in risk profiles of the two patient populations analyzed. An immune-mediated primary regression in MUP as previously discussed may indicate a higher immune competence with enhanced immune responses against tumor cells in MUP patients. As reported by Scott et al.5 regressing melanomas are characterized by increased numbers of tumor-infiltrating lymphocytes, which also confer a favorable prognosis. Immune responses to melanoma-associated antigens are also mediated through cytotoxic T-lymphocytes.5 In addition, Scott et al.5 reported that there is a high prevalence of melanoma-specific antibodies in the serum of MUP patients, and various humoral immune mechanisms, including antibody attachment to cell membranes, cytotoxicity, and tumor destruction, have all been described with cultured melanoma cells.5 Possibly, MUP patients may be excellent candidates for ICPI therapies due to enhanced capabilities to orchestrate anti-tumor immune responses under ICPI treatment.
One may argue, however, that the better outcome observed in MUP patients was based on an anyway more favorable survival of MUP patients when compared MKP patients. Indeed, as already mentioned in the introduction section the literature in this regard is controversial as also reflected by the aforementioned results reported by Utter et al.6 and Verver et al.6,16 Prior to the start of ICIT, all of our MUP patients were at stage IV disease requiring systemic therapy. Scott et al.,8 recently reported that the incidence of stage IV MUP is increasing. Whereas the relative survival was higher for MUP, the 5-year adjusted melanoma-specific survival was equal for MUP and MKP. Schlagenhauff et al.23 also showed that the course of disease of MUP is similar to that of MKP. With respect to stage III disease, Hughes et al.14 demonstrated that patients who underwent lymphadenectomy for stage III melanomas of the known or unknown primary site do not differ in the outcome. However, other authors previously reported on a better outcome for MUP compared to MKP.11,15,24,25
In conclusion, the results of the present pilot study with inherent limitations such as low numbers of included patients are just preliminary but highly suggestive that MUP patients have less risk for MSD on ICPI therapy. However, this needs confirmation in prospective studies including large sample sizes.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- 1.Prado G, Svoboda RM, Rigel DS.. What’s new in melanoma. Dermatol Clin. 2019;37(2):1–5. doi: 10.1016/j.det.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Carreau NA, Pavlick AC.. Nivolumab and ipilimumab: immunotherapy for treatment of malignant melanoma. Future Oncol. 2019;15:349–358. doi: 10.2217/fon-2018-0607. [DOI] [PubMed] [Google Scholar]
- 3.Tos T, Klyver H, Drzewiecki KT. Extensive screening for primary tumor is redundant in melanoma of unknown primary. J Surg Oncol. 2011;104(7):724–727. doi: 10.1002/jso.21994. [DOI] [PubMed] [Google Scholar]
- 4.Chantharasamee J, Treetipsatit J. Metastatic melanoma of uncertain primary with 5-year durable response after conventional therapy: a case report with literature review. Case Rep Oncol Med. 2018. May 31;2018:7289896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott JF, Gerstenblith MR. Melanoma of unknown primary In: Scott JF, Gerstenblith MR, editors. Noncutaneous melanoma [Internet]. Brisbane (AU): Codon Publications; 2018. March Chapter 7. PMID: 29874016. http://www.ncbi.nlm.nih.gov/books/NBK506989/PubMed. [PubMed] [Google Scholar]
- 6.Utter K, Goldman C, Weiss SA, Shapiro RL, Berman RS, Wilson MA, Pavlick AC, Osman I. Treatment outcomes for metastatic melanoma of unknown primary in the new era: a single-institution study and review of the literature. Oncology. 2017;93(4):249–258. doi: 10.1159/000478050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunssen A, Jansen L, Eisemann N, Waldmann A, Weberpals J, Kraywinkel K, Eberle A, Holleczek B, Zeissig SR, Brenner H, et al. Long-term relative survival from melanoma in Germany 1997-2013. Melanoma Res. 2018. July 16;1. doi: 10.1097/CMR.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 8.Scott JF, Conic RZ, Thompson CL, Gerstenblith MR, Bordeaux JS. Stage IV melanoma of unknown primary: A population-based study in the United States from 1973 to 2014. J Am Acad Dermatol. 2018. August;79(2):258–265.e4. doi: 10.1016/j.jaad.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribero S, Pampena R, Bataille V, Moscarella E, Thomas L, Quaglino P, Potenza C, Van Akkooi ACJ, Testori A, Nathan P, et al. Unknown primary melanoma: worldwide survey on clinical management. Dermatology. 2016;232(6):704–707. doi: 10.1159/000453592. [DOI] [PubMed] [Google Scholar]
- 10.Kuk D, Shoushtari AN, Barker CA, Panageas KS, Munhoz RR, Momtaz P, Ariyan CE, Brady MS, Coit DG, Bogatch K, et al. Prognosis of mucosal, uveal, acral, nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist. 2016. July;21(7):848–854. doi: 10.1634/theoncologist.2015-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae JM, Choi YY, Kim DS, Lee JH, Jang HS, Lee JH, Kim H, Oh BH, Roh MR, Nam KA, et al. Metastatic melanomas of unknown primary show better prognosis than those of known primary: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2015. January;72(1):59–70. doi: 10.1016/j.jaad.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Egberts F, Bergner I, Krüger S, Haag J, Behrens HM, Hauschild A, Röcken C. Metastatic melanoma of unknown primary resembles the genotype of cutaneous melanomas. Ann Oncol. 2014. January;25(1):246–250. doi: 10.1093/annonc/mdt411. [DOI] [PubMed] [Google Scholar]
- 13.Weide B, Faller C, Elsässer M, Büttner P, Pflugfelder A, Leiter U, Eigentler TK, Bauer J, Meier F, Garbe C. Melanoma patients with unknown primary site or nodal recurrence after initial diagnosis have a favourable survival compared to those with synchronous lymph node metastasis and primary tumour. PLoS One. 2013. June 25;8(6):e66953. doi: 10.1371/journal.pone.0066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes MC, Wright A, Barbour A, Thomas J, Smithers BM, Green AC, Khosrotehrani K. Patients undergoing lymphadenectomy for stage III melanomas of known or unknown primary site do not differ in outcome. Int J Cancer. 2013. December 15;133(12):3000–3007. doi: 10.1002/ijc.28318. [DOI] [PubMed] [Google Scholar]
- 15.van der Ploeg AP, Haydu LE, Spillane AJ, Scolyer RA, Quinn MJ, Saw RP, Shannon KF, Stretch JR, Thompson JF. Melanoma patients with an unknown primary tumor site have a better outcome than those with a known primary following therapeutic lymph node dissection for macroscopic (clinically palpable) nodal disease. Ann Surg Oncol. 2014. September;21(9):3108–3116. doi: 10.1245/s10434-014-3679-5. [DOI] [PubMed] [Google Scholar]
- 16.Verver D, van der Veldt A, van Akkooi A, Verhoef C, Grünhagen DJ, Louwman WJ. Treatment of melanoma of unknown primary in the era of immunotherapy and targeted therapy: a Dutch population-based study. Int J Cancer. 2019. February 23. doi: 10.1002/ijc.32229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y, Karakousis GC. Melanoma of unknown primary. J Surg Oncol. 2019. January;119(2):232–241. doi: 10.1002/jso.25302. [DOI] [PubMed] [Google Scholar]
- 18.Dasgupta T, Bowden L, Berg JW. Malignant melanoma of unknown primary origin. Surg Gynecol Obstet. 1963;117:341–345. [PubMed] [Google Scholar]
- 19.Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur GA, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kähler KC, Hassel JC, Heinzerling L, Loquai C, Mössner R, Ugurel S, Zimmer L, Gutzmer R “Cutaneous Side Effects” Committee of the Work Group Dermatological Oncology (ADO). J Dtsch Dermatol Ges. 2016;14:662–681. [DOI] [PubMed] [Google Scholar]
- 21.Pan M, Alavi M, Herrinton LJ. Association of inflammatory markers with disease progression in patients with metastatic melanoma treated with immune checkpoint inhibitors. Perm J. 2018;22:17–149. doi: 10.7812/TPP/17-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felix J, Cassinat B, Porcher R, Schlageter M-H, Maubec E, Pages C, Baroudjian B, Homyrda L, Boukouaci W, Tamouza R, et al. Relevance of serum biomarkers associated with melanoma during follow-up of anti-CTLA-4 immunotherapy. Int Immunopharmacol. 2016;40:466–473. doi: 10.1016/j.intimp.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 23.Schlagenhauff B, Stroebel W, Ellwanger U, Meier F, Zimmermann C, Breuninger H, Rassner G, Garbe C. Metastatic melanoma of unknown primary origin shows prognostic similarities to regional metastatic melanoma: recommendations for initial staging examinations. Cancer. 1997. July 1;80(1):60–65. doi:. [DOI] [PubMed] [Google Scholar]
- 24.Prens SP, van der Ploeg AP, van Akkooi AC, van Montfort CA, van Geel AN, de Wilt JH, Eggermont AM, Verhoef C. Outcome after therapeutic lymph node dissection in patients with unknown primary melanoma site. Ann Surg Oncol. 2011;18:3586–3592. doi: 10.1245/s10434-011-1801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CC, Faries MB, Wanek LA, Morton DL. Improved survival for stage IV melanoma from an unknown primary site. J Clin Oncol. 2009;27:3489–3495. doi: 10.1200/JCO.2008.18.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]


