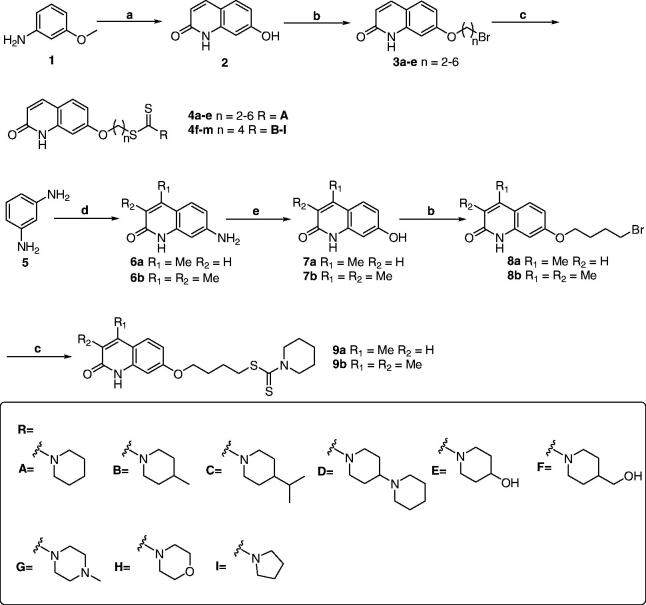
Scheme 1.
Synthesis of compounds 4a-m and 9a-b. Reagents and conditions: (a) (i) cinnamoyl chloride, dry dichloromethane, 4 h, reflux; (ii) AlCl3, chlorobenzene, 8 h, reflux; (b) α, ω-dibromoalkanes, K2CO3, acetone, reflux, 4 h; (c) appropriate secondary amines, CS2, TEA, DMF, r.t., 12 h. (d) ethyl acetoacetate for 6a, ethyl 2-methylacetoacetate for 6 b, 150 °C, 48 h; (e) (i) NaNO2, H2SO4, 0 °C; (ii) 10 M, H2SO4, reflux, 10 min.