Figure 5.
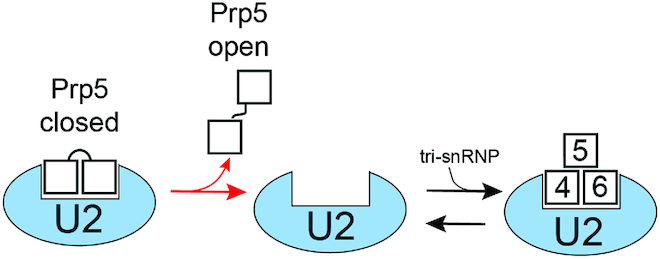
Potential Role for Prp5 Conformational Switching during Spliceosome Assembly. Release of Prp5 from U2 could be accompanied by spontaneous transition from the closed to the open conformation of the Prp5 RecA domains. Since the open conformation is stable in the absence of both RNA and ATP, this could render Prp5 release effectively irreversible (red forward arrow) and facilitate subsequent tri-snRNP binding. For simplicity, we have shown Prp5 and the tri-snRNP with overlapping binding sites on U2. While it is known that Prp5 blocks tri-snRNP addition (13), a structure of Prp5 bound to U2 has not yet been determined, nor is it known if the U2 snRNP undergoes conformational change after Prp5 release.
