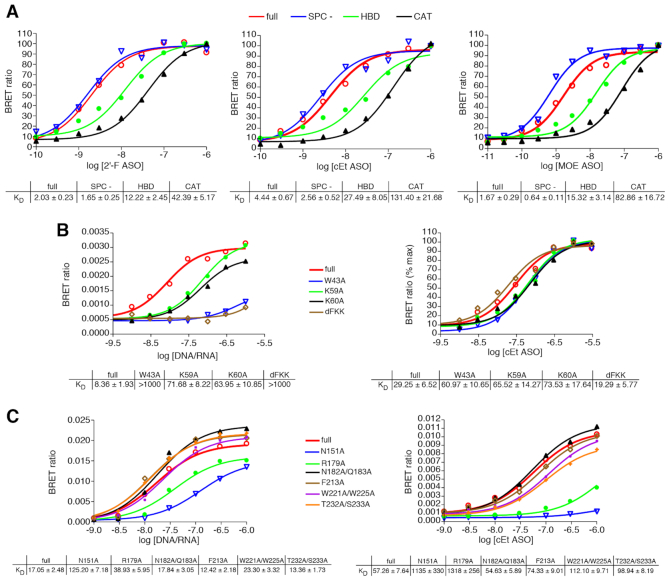
Figure 7.
(A) ASOs bind RNAse H1 at the HBD and catalytic domains, but do not interact with the spacer domain. NLuc-RNAse H1 fusion proteins were immunopurified as detailed in Online Methods and subsequently incubated with Alexa 594 conjugated 5-10-5 cEt gap-mer ASO at concentrations ranging from 10 pM to 1 μM. BRET ratios were determined for full-length NLuc-RNAse H1 (red), Spacer- (blue), HBD-only (green) or CAT-only (black). Concentration response curves and KD’s ± SEM (nM) are representative of 2–3 three independent experiments. B) PS-ASOs interact with the RNAse H1 HBD differently than does the native RNA/DNA heteroduplex. ASO/BRET assays were carried out with purified NLuc-RNAse H1 HBD fusion protein and with the indicated HBD mutant proteins. The purified fusion proteins were incubated with Alexa 594 conjugated 5-10-5 cEt gap-mer ASO or with DNA/RNA heteroduplex of the same sequence with Alexa 594 on the DNA strand at concentrations ranging from 10 pM to 3 μM. Concentration response curves and KD’s ± SEM (nM) are representative of two independent experiments. (C) PS-ASOs and PO heteroduplex interact with the RNase H1 catalytic domain similarly. ASO/BRET assays were carried out with purified NLuc-RNase H1 CAT fusion protein and with the indicated CAT domain mutant proteins. The purified fusion proteins were incubated with Alexa 594 conjugated 5-10-5 cEt gap-mer ASO or with DNA/RNA heteroduplex as above at concentrations ranging from 10 pM to 1 μM. Concentration response curves and KD’s ± SEM (nM) are representative of 2 independent experiments.