Figure 2.
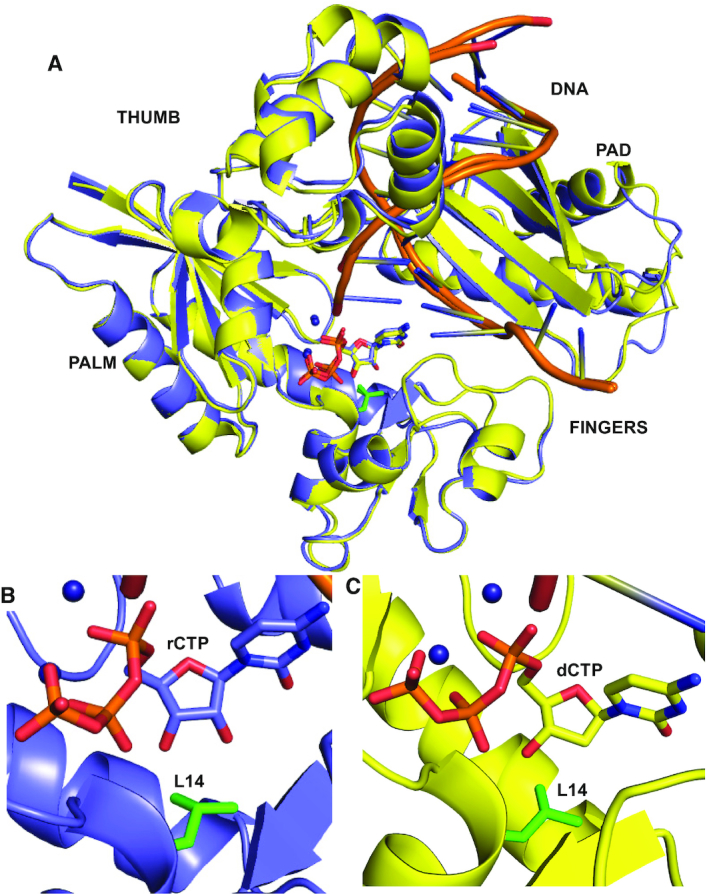
Structural mechanism of rCTP and dCTP incorporation by MsDpo4-WT. (A) The structures of MsDpo4-WTDNA(dG):dCTP (colored yellow) and MsDpo4-WTDNA(dG):rCTP (colored violet) superimpose with an RMSD of 0.36 Å. The enzyme structure is shown in cartoon representation, DNA is shown as ribbons, and the incoming nucleotide and the Leu14 residue (in green) are shown in stick representation. A close-up of the region surrounding the incoming nucleotide, is displayed for (B) MsDpo4DNA(dG):rCTP and (C) MsDpo4DNA(dG):dCTP. The comparison shows that the dCTP and rCTP bind the MsDpo4 active site in the same location and conformation with marginal differences in the enzyme structure.
