Figure 7.
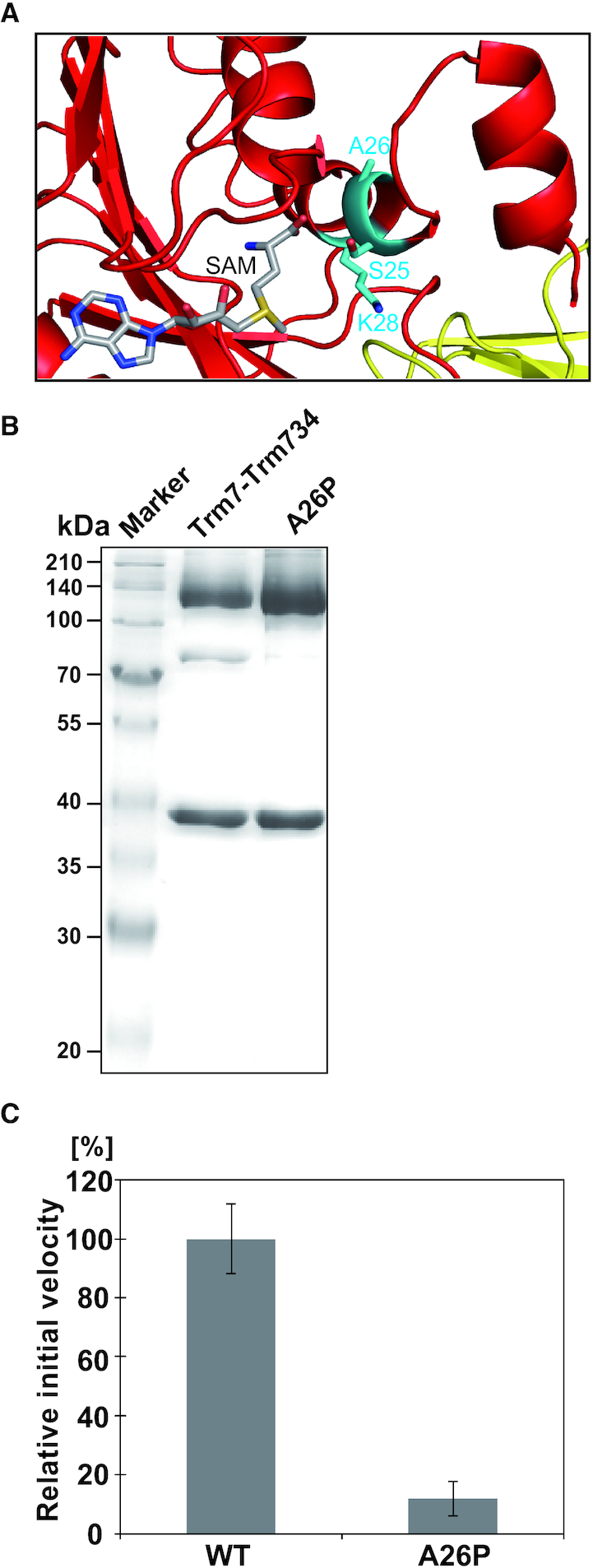
Activity of the A26P mutant. (A) Close-up view of the peripheral structure of α helix (α2) and SAM in Trm7. The residues, S25 A26 and K28 are shown as stick models (cyan). SAM is illustrated as a stick model. (B) About 12% SDS-PAGE of purified A26P mutant. The gel was stained with Coomassie Brilliant Blue. (C) Relative methyl-transfer activities of the wild-type Trm7–Trm734 (WT) and A26P mutant. The initial velocity of the WT for tRNAPhe transcript is expressed as 100.0%.
