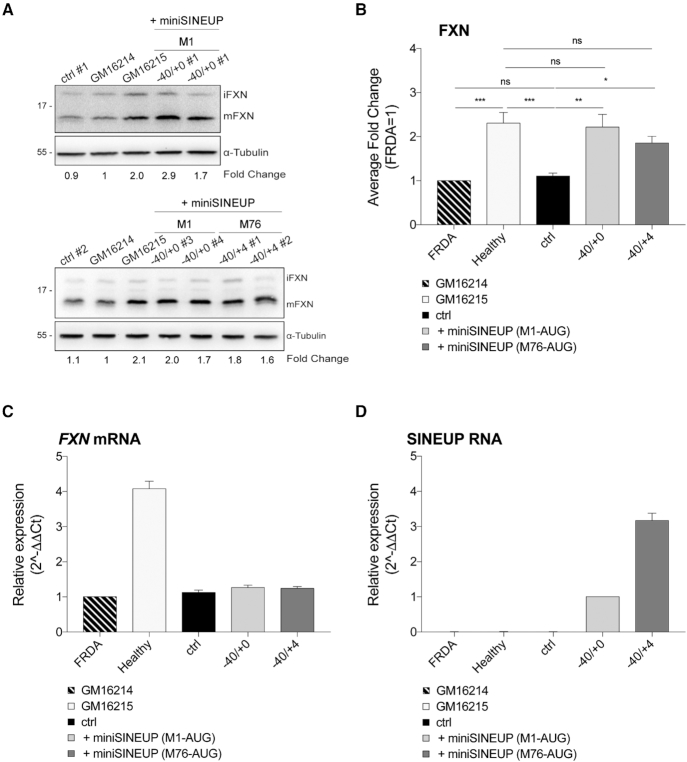
Figure 7.
Rescue of frataxin protein levels in FRDA patient-derived lymphoblasts. (A-D) GM16214 cells (patients’ primary lymphoblasts) were stably transfected with miniSINEUP-FXN variants and empty vector (ctrl). miniSINEUP-FXN (−40/+0 M1-AUG), miniSINEUP-FXN (−40/+4 M76-AUG) and ctrl stable clones were obtained from at least 15 days of G418 selection. (A) Whole cell lysates were analysed by western blotting with anti-FXN and anti-β-actin antibodies. Two representative experiments are shown. First, FXN band intensity was normalized to the relative β-actin. Then, fold change values were calculated normalizing to GM16214 cells. GM16214 cells expressing miniSINEUP-FXN show increased levels of endogenous FXN protein. (B) Average fold change of FXN protein levels. Columns represent mean ± S.E.M. of n ≥ 4 independent experiments; ns, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA followed by Dunnett's post-test). (C, D) The real-time RT-PCR analysis of FXN mRNA and miniSINEUP RNA expression in transfected cells. Columns represent mean ± S.E.M. of n≥4 independent experiments. Variation in both target and miniSINEUP mRNA expression among samples are not statistically significant (one-way ANOVA followed by Dunnett's post-test). (C) FXN transcripts were quantified, using human GAPDH (hGAPDH) expression as the internal control. The FXN/hGAPDH ratio for the GM16214 sample was set as a baseline value to which all transcripts levels were normalized. Unchanged FXN mRNA levels are shown. (D) miniSINEUP transcripts were quantified, using the hGAPDH expression as the internal control. The miniSINEUP/hGAPDH ratio for −40/+0 M1-AUG sample was set as a baseline value to which all transcripts levels were normalized.