Figure 2.
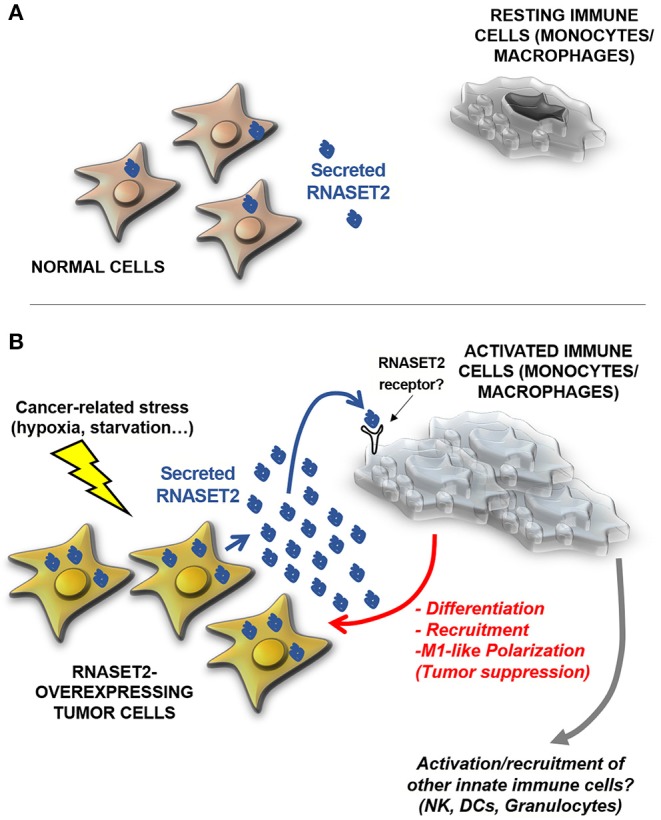
A model for human RNASET2-mediated tumor suppression. (A) In physiological contexts, most human cells express low or undetectable RNASET2 levels (https://www.ncbi.nlm.nih.gov/gene/8635), with the notable exception of spleen, lymph nodes and colon, three tissues highly involved in immune system function. This ubiquitous “baseline” RNASET2 expression can be related to the execution of intracellular or extracellular roles (some yet-to-be defined) possibly mediated by its catalytic activity. (B) When cells are locally exposed to a wide range of stresses, some of which are typically experienced by cancer cells (such as hypoxia, oxidative stress or nutritional starvation), they activate a “danger-response” program which involves, besides the activation of several endogenous stress response pathways, a massive increase in expression and secretion of RNASET2, which acts as an alarmin-like molecule to engage cells belonging to the innate immune system (mostly macrophages, but possibly other cellular components such as natural killer (NKs), dendritic cells (DCs) and granulocytes) to coordinate an immune-response-mediated tumor suppressive response. As previously described for several biological processes mediated by T2 RNases, the catalytic activity of the RNASET2 is not required to trigger this marked immune system-mediated response. Strikingly, the functional crosstalk between RNASET2 and cellular effectors of the innate immune system has been reported in several evolutionary distant species, suggesting an ancient key role of T2 RNases in immune-response mediated host defense.
