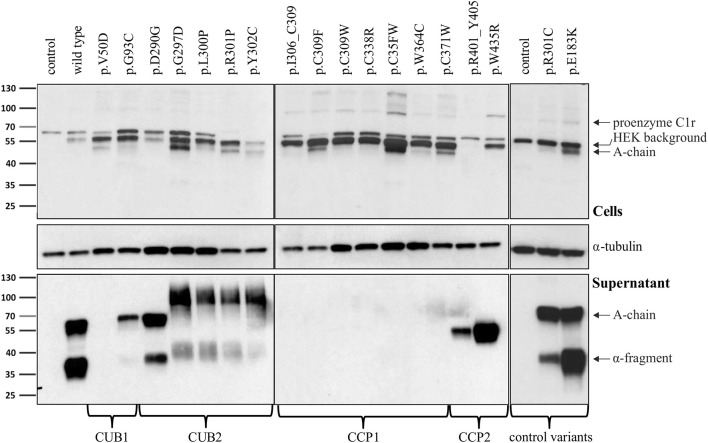
Figure 2.
Domain-specific secretion patterns of C1r variant proteins. HEK293T cells were transiently transfected with C1r WT or variants (“control” = empty vector). Cell lysates (upper panel) and supernatants (lower panel) were used for western blot with an N-terminal anti-C1r antibody 48 h after transfection under reducing conditions. This antibody may specifically visualize (1) the full-length C1r (100 kDa); (2) the A-chain (55 kDa) after auto-activation of C1r; or (3) the α-fragment (35 kDa) generated via an auto-proteolytic event after secretion of the activated protein. An intracellular HEK-specific background band is seen at around 60 kDa. Little full-length C1r (100 kDa) and presence of A-chain was detected in all cell lysates. Most variants resulted in increased intracellular presence of A-chain fragments, sometimes with an additional smaller C1r fragment that may represent a degradation product. Overexpression of C1r WT, the control variants p.(R301C) and p.(E183K), and the variant p.(D290G), resulted in high amounts of A-chain and α-fragment in the supernatant. All other variants show domain-specific abnormalities of intracellular processing and secretion. Strongly reduced extracellular secretion was found for variants in the CUB1 and CCP1 domains. All CUB2 variants (except p.(D290G)) show abnormal protein aggregates in the supernatant, the exact nature of which cannot be specified. Abnormal protein fragments without proteolysis were found for CCP2 variants.