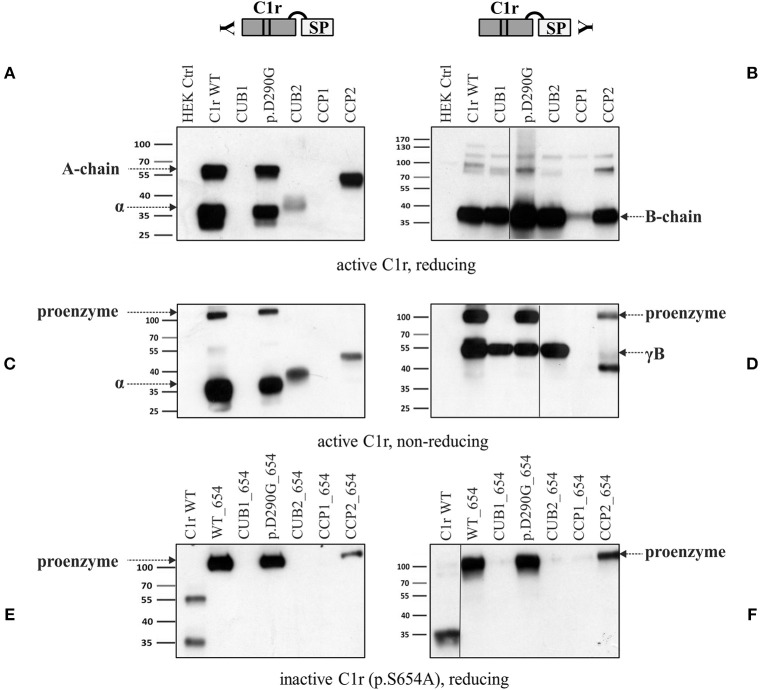
Figure 4.
Secretion of C1r active serine protease for all C1r variants. HEK293T cells were transiently transfected with C1r WT or variant as enzymatically active or inactive (additional mutation p.S654A) form (“HEK Ctrl” = empty vector). Supernatant 48 h post-transfection was used for western blot with N- and C-terminal antibodies under reducing or non-reducing conditions as indicated. One mutation of each domain was chosen: CUB1 (p.V50D), C1q-binding site (p.(D290G)), CUB2 (p.(L300P)), CCP1 (p.(C338R)), CCP2 (p.W435R). (A) Domain-specific secretion pattern of N-terminal fragments. (B) C1r WT and all variants show secretion of catalytic B-chain. (C) Shows same domain-specific pattern as in (A) with expected differences (disulfide-linked chains running as full-length protein) under non-reducing conditions. (D) Presence of full-length in WT, p.(D290G) and CCP2. The γB-fragment is present in WT and all variants with the exception of CCP1. The new cleavage site in CCP2 variant results in smaller fragment size. (E,F) Enzymatically inactive C1r variants were secreted for WT, p.(D290G), and CCP2. Other variants were not detected in the supernatant.